Investigating the elusive mechanism of glycosaminoglycan biosynthesis
- PMID: 19628873
- PMCID: PMC2757986
- DOI: 10.1074/jbc.M109.043208
Investigating the elusive mechanism of glycosaminoglycan biosynthesis
Abstract
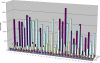
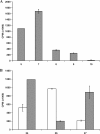
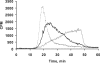
Glycosaminoglycan (GAG) biosynthesis requires numerous biosynthetic enzymes and activated sulfate and sugar donors. Although the sequence of biosynthetic events is resolved using reconstituted systems, little is known about the emergence of cell-specific GAG chains (heparan sulfate, chondroitin sulfate, and dermatan sulfate) with distinct sulfation patterns. We have utilized a library of click-xylosides that have various aglycones to decipher the mechanism of GAG biosynthesis in a cellular system. Earlier studies have shown that both the concentration of the primers and the structure of the aglycone moieties can affect the composition of the newly synthesized GAG chains. However, it is largely unknown whether structural features of aglycone affect the extent of sulfation, sulfation pattern, disaccharide composition, and chain length of GAG chains. In this study, we show that aglycones can switch not only the type of GAG chains, but also their fine structures. Our findings provide suggestive evidence for the presence of GAGOSOMES that have different combinations of enzymes and their isoforms regulating the synthesis of cell-specific combinatorial structures. We surmise that click-xylosides are differentially recognized by the GAGOSOMES to generate distinct GAG structures as observed in this study. These novel click-xylosides offer new avenues to profile the cell-specific GAG chains, elucidate the mechanism of GAG biosynthesis, and to decipher the biological actions of GAG chains in model organisms.
Figures

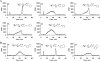




References
-
- Sasisekharan R., Shriver Z., Venkataraman G., Narayanasami U. (2002) Nat. Rev. Cancer 2, 521–528 - PubMed
-
- Lander A. D. (1993) Curr. Opin. Neurobiol. 3, 716–723 - PubMed
-
- Capila I., Linhardt R. J. (2002) Angew. Chem. Int. Ed. Engl. 41, 391–412 - PubMed
-
- Powell A. K., Yates E. A., Fernig D. G., Turnbull J. E. (2004) Glycobiology 14, 17R–30R - PubMed
-
- Salmivirta M., Lidholt K., Lindahl U. (1996) FASEB J. 10, 1270–1279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

