Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism
- PMID: 19628874
- PMCID: PMC2757961
- DOI: 10.1074/jbc.M109.025452
Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism
Erratum in
- J Biol Chem. 2012 Feb 3;287(6):4394
Abstract
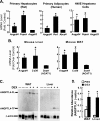
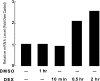
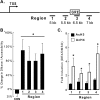
Glucocorticoids are important regulators of lipid homeostasis, and chronically elevated glucocorticoid levels induce hypertriglyceridemia, hepatic steatosis, and visceral obesity. The occupied glucocorticoid receptor (GR) is a transcription factor. However, those genes regulating lipid metabolism under GR control are not fully known. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor), a protein inhibitor of lipoprotein lipase, is synthesized and secreted during fasting, when circulating glucocorticoid levels are physiologically increased. We therefore tested whether the ANGPTL4 gene (Angptl4) is transcriptionally controlled by GR. We show that treatment with the synthetic glucocorticoid dexamethasone increased Angptl4 mRNA levels in primary hepatocytes and adipocytes (2-3-fold) and in the livers and white adipose tissue of mice (approximately 4-fold). We tested the mechanism of this increase in H4IIE hepatoma cells and found that dexamethasone treatment increased the transcriptional rate of Angptl4. Using bioinformatics and chromatin immunoprecipitation, we identified a GR binding site within the rat Angptl4 sequence. A reporter plasmid containing this site was markedly activated by dexamethasone, indicative of a functional glucocorticoid response element. Dexamethasone treatment also increased histone H4 acetylation and DNase I accessibility in genomic regions near this site, further supporting that it is a glucocorticoid response element. Glucocorticoids promote the flux of triglycerides from white adipose tissue to liver. We found that mice lacking ANGPTL4 (Angptl4(-/-)) had reductions in dexamethasone-induced hypertriglyceridemia and hepatic steatosis, suggesting that ANGPTL4 is required for this flux. Overall, we establish that ANGPTL4 is a direct GR target that participates in glucocorticoid-regulated triglyceride metabolism.
Figures


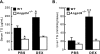
References
-
- Macfarlane D. P., Forbes S., Walker B. R. (2008) J. Endocrinol. 197, 189–204 - PubMed
-
- Seckl J. R., Morton N. M., Chapman K. E., Walker B. R. (2004) Recent Prog. Horm. Res. 59, 359–393 - PubMed
-
- Hato T., Tabata M., Oike Y. (2008) Trends Cardiovasc. Med. 18, 6–14 - PubMed
-
- Kersten S. (2005) Biochem. Soc. Trans. 33, 1059–1062 - PubMed
-
- Li C. (2006) Curr. Opin. Lipidol. 17, 152–156 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

