A 'higher order' of telomere regulation: telomere heterochromatin and telomeric RNAs
- PMID: 19629032
- PMCID: PMC2722253
- DOI: 10.1038/emboj.2009.197
A 'higher order' of telomere regulation: telomere heterochromatin and telomeric RNAs
Abstract
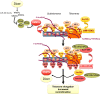
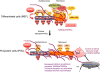
Protection of chromosome ends from DNA repair and degradation activities is mediated by specialized protein complexes bound to telomere repeats. Recently, it has become apparent that epigenetic regulation of the telomeric chromatin template critically impacts on telomere function and telomere-length homeostasis from yeast to man. Across all species, telomeric repeats as well as the adjacent subtelomeric regions carry features of repressive chromatin. Disruption of this silent chromatin environment results in loss of telomere-length control and increased telomere recombination. In turn, progressive telomere loss reduces chromatin compaction at telomeric and subtelomeric domains. The recent discoveries of telomere chromatin regulation during early mammalian development, as well as during nuclear reprogramming, further highlights a central role of telomere chromatin changes in ontogenesis. In addition, telomeres were recently shown to generate long, non-coding RNAs that remain associated to telomeric chromatin and will provide new insights into the regulation of telomere length and telomere chromatin. In this review, we will discuss the epigenetic regulation of telomeres across species, with special emphasis on mammalian telomeres. We will also discuss the links between epigenetic alterations at mammalian telomeres and telomere-associated diseases.
Figures

References
-
- Abad JP, De Pablos B, Osoegawa K, De Jong PJ, Martin-Gallardo A, Villasante A (2004) Genomic analysis of Drosophila melanogaster telomeres: full-length copies of HeT-A and TART elements at telomeres. Mol Biol Evol 21: 1613–1619 - PubMed
-
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev 9: 218–233 - PubMed
-
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL (2003) Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 102: 517–520 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

