The life and miracles of kinetochores
- PMID: 19629042
- PMCID: PMC2722247
- DOI: 10.1038/emboj.2009.173
The life and miracles of kinetochores
Abstract
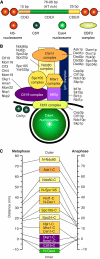
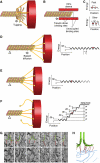
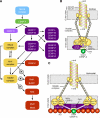
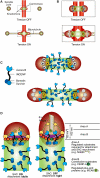
Kinetochores are large protein assemblies built on chromosomal loci named centromeres. The main functions of kinetochores can be grouped under four modules. The first module, in the inner kinetochore, contributes a sturdy interface with centromeric chromatin. The second module, the outer kinetochore, contributes a microtubule-binding interface. The third module, the spindle assembly checkpoint, is a feedback control mechanism that monitors the state of kinetochore-microtubule attachment to control the progression of the cell cycle. The fourth module discerns correct from improper attachments, preventing the stabilization of the latter and allowing the selective stabilization of the former. In this review, we discuss how the molecular organization of the four modules allows a dynamic integration of kinetochore-microtubule attachment with the prevention of chromosome segregation errors and cell-cycle progression.
Figures





References
-
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

