Synthesis and combinational antibacterial study of 5''-modified neomycin
- PMID: 19629142
- PMCID: PMC2783947
- DOI: 10.1038/ja.2009.66
Synthesis and combinational antibacterial study of 5''-modified neomycin
Abstract
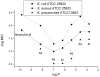
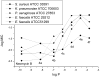
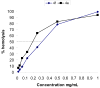
A library of 5''-modified neomycin derivatives were synthesized for an antibacterial structure-activity optimization strategy. Two leads exhibited prominent activity against both methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). Antibacterial activities were measured when combined with other clinically used antibiotics. Significant synergistic activities were observed, which may lead to the development of novel therapeutic practices in the battle against infectious bacteria.
Figures
References
-
- Umezawa S, Tsuchiya T. In: Aminoglycoside Antibiotics. Umezawa H, Hooper IR, editors. Springer-Verlag; New York: 1982. pp. 37–110.
-
- Haddad J, Kotra LP, Mobashery S. In: Glycochemistry Principles, Synthesis, and Applications. Wang PG, Bertozzi CR, editors. Marcel Dekker, Inc.; New York/Basel: 2001. pp. 353–424.
-
- Wang J, Chang CWT. In: Aminoglycoside Antibiotics. Arya DP, editor. John Wiley & Sons, Inc.; 2007. pp. 141–180.
-
- Wang J, Li J, Chen HN, Chang H, Tanifum CT, Liu HH, Czyryca PG, Chang CWT. Glycodiversification for optimization of the kanamycin class aminoglycosides. J Med Chem. 2005;48:6271–6285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases