Power efficiency of outer hair cell somatic electromotility
- PMID: 19629162
- PMCID: PMC2705677
- DOI: 10.1371/journal.pcbi.1000444
Power efficiency of outer hair cell somatic electromotility
Abstract
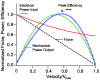
Cochlear outer hair cells (OHCs) are fast biological motors that serve to enhance the vibration of the organ of Corti and increase the sensitivity of the inner ear to sound. Exactly how OHCs produce useful mechanical power at auditory frequencies, given their intrinsic biophysical properties, has been a subject of considerable debate. To address this we formulated a mathematical model of the OHC based on first principles and analyzed the power conversion efficiency in the frequency domain. The model includes a mixture-composite constitutive model of the active lateral wall and spatially distributed electro-mechanical fields. The analysis predicts that: 1) the peak power efficiency is likely to be tuned to a specific frequency, dependent upon OHC length, and this tuning may contribute to the place principle and frequency selectivity in the cochlea; 2) the OHC power output can be detuned and attenuated by increasing the basal conductance of the cell, a parameter likely controlled by the brain via the efferent system; and 3) power output efficiency is limited by mechanical properties of the load, thus suggesting that impedance of the organ of Corti may be matched regionally to the OHC. The high power efficiency, tuning, and efferent control of outer hair cells are the direct result of biophysical properties of the cells, thus providing the physical basis for the remarkable sensitivity and selectivity of hearing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
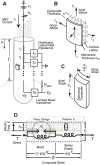
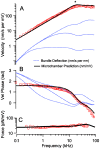
 to give the composite strain. The base of the cell was modeled as a simple membrane with conductance and capacitance per unit area (Zb in panel A). Four stimulus conditions were simulated: sinusoidal transduction current injection IT entering the apical pole of the cell, sinusoidal displacement of the hair bundle leading to frequency-dependent MET currents, sinusoidal voltage clamp of the intracellular voltage Vb at the base using a patch pipette and, sinusoidal modulation of the extracellular voltage Vp at the base using a glass microchamber sealed with resistance Rseal around the passive basal pole of the cell. In all simulations, cells were held stationary at
to give the composite strain. The base of the cell was modeled as a simple membrane with conductance and capacitance per unit area (Zb in panel A). Four stimulus conditions were simulated: sinusoidal transduction current injection IT entering the apical pole of the cell, sinusoidal displacement of the hair bundle leading to frequency-dependent MET currents, sinusoidal voltage clamp of the intracellular voltage Vb at the base using a patch pipette and, sinusoidal modulation of the extracellular voltage Vp at the base using a glass microchamber sealed with resistance Rseal around the passive basal pole of the cell. In all simulations, cells were held stationary at  and generated force or movement at
and generated force or movement at  .
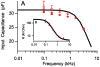
.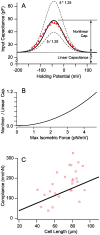
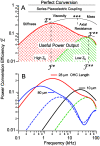
 occurs at a best frequency
occurs at a best frequency  , and shifts down in magnitude and up in frequency with opening of conductive ion channels in the basal cell membrane (
, and shifts down in magnitude and up in frequency with opening of conductive ion channels in the basal cell membrane ( ,
,  ). Hence, shunting of the basal impedance by efferent action on OHCs is predicted to attenuate their power output at best frequency
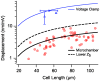
). Hence, shunting of the basal impedance by efferent action on OHCs is predicted to attenuate their power output at best frequency  , by well over an order of magnitude. B) The most efficient frequency depends upon cell length. Shorter cells show peak efficiencies at higher frequencies (10 µm) while longer cells show peak efficiencies at lower frequencies (80 µm). These predictions were computed by adjusting the load to be impedance matched at each frequency (peak efficiency load in Fig. 6 denoted by *).
, by well over an order of magnitude. B) The most efficient frequency depends upon cell length. Shorter cells show peak efficiencies at higher frequencies (10 µm) while longer cells show peak efficiencies at lower frequencies (80 µm). These predictions were computed by adjusting the load to be impedance matched at each frequency (peak efficiency load in Fig. 6 denoted by *).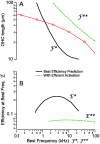
References
-
- Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. - PubMed
-
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64 - PubMed
-
- Rhode WS. Observations of the vibration of the basilare membrane in squirrel monkeys using the Mossbauer technique. J Acoust Soc Am. 1971;49 - PubMed
-
- Mountain DC. Changes in endolymphatic potential and crossed olivo cochlear bundle stimulation alter cochlear mechanics. Science. 1980;210:71–72. - PubMed
-
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

