Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis
- PMID: 19629173
- PMCID: PMC2709079
- DOI: 10.1371/journal.pcbi.1000445
Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis
Abstract
The extracellular matrix plays a critical role in orchestrating the events necessary for wound healing, muscle repair, morphogenesis, new blood vessel growth, and cancer invasion. In this study, we investigate the influence of extracellular matrix topography on the coordination of multi-cellular interactions in the context of angiogenesis. To do this, we validate our spatio-temporal mathematical model of angiogenesis against empirical data, and within this framework, we vary the density of the matrix fibers to simulate different tissue environments and to explore the possibility of manipulating the extracellular matrix to achieve pro- and anti-angiogenic effects. The model predicts specific ranges of matrix fiber densities that maximize sprout extension speed, induce branching, or interrupt normal angiogenesis, which are independently confirmed by experiment. We then explore matrix fiber alignment as a key factor contributing to peak sprout velocities and in mediating cell shape and orientation. We also quantify the effects of proteolytic matrix degradation by the tip cell on sprout velocity and demonstrate that degradation promotes sprout growth at high matrix densities, but has an inhibitory effect at lower densities. Our results are discussed in the context of ECM targeted pro- and anti-angiogenic therapies that can be tested empirically.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
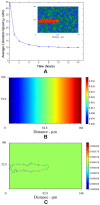
 . Above
. Above  , extension speeds are significantly reduced and for
, extension speeds are significantly reduced and for  and
and  normal angiogenesis is interrupted suggesting that modulating matrix density may be an effective anti-angiogenesis therapy. (B) Quantification of morphological properties of the sprout showing sprout thicknesses within normal physiological ranges but dependent on matrix density and a distinct range of fiber density conductive to branching.
normal angiogenesis is interrupted suggesting that modulating matrix density may be an effective anti-angiogenesis therapy. (B) Quantification of morphological properties of the sprout showing sprout thicknesses within normal physiological ranges but dependent on matrix density and a distinct range of fiber density conductive to branching.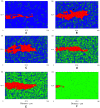
 , interruption of normal angiogenesis and loss of sprout viability; (B)
, interruption of normal angiogenesis and loss of sprout viability; (B)  , high matrix heterogeneity induces branching (arrow points to new branch); (C)
, high matrix heterogeneity induces branching (arrow points to new branch); (C)  , anastomosis; (D)
, anastomosis; (D)  , more homogeneous matrix fiber network produces linear sprouts; (E)
, more homogeneous matrix fiber network produces linear sprouts; (E)  , higher matrix homogeneity causes loss of strong guidance cues resulting in wider and slower sprout formation; and (F)
, higher matrix homogeneity causes loss of strong guidance cues resulting in wider and slower sprout formation; and (F)  , complete inhibition of angiogenesis at high matrix density. Snapshots at 14 hours.
, complete inhibition of angiogenesis at high matrix density. Snapshots at 14 hours.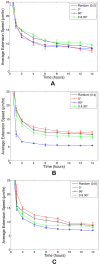
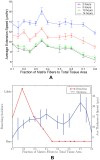
 , fiber network is not well connected and matrix fiber alignment does not have a strong effect on sprout extension speeds. At
, fiber network is not well connected and matrix fiber alignment does not have a strong effect on sprout extension speeds. At  (B) and
(B) and  (C), rates of sprout extension are more rapid when matrix fibers are aligned parallel to VEGF gradients (0°) than when matrix fibers are aligned perpendicular to the gradient (90°).
(C), rates of sprout extension are more rapid when matrix fibers are aligned parallel to VEGF gradients (0°) than when matrix fibers are aligned perpendicular to the gradient (90°).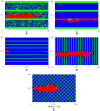
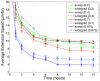
 , matrix degradation has anti-angiogenic effects. Above
, matrix degradation has anti-angiogenic effects. Above  , degradation facilitates sprout progression.
, degradation facilitates sprout progression.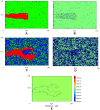
 by carving out a path for migration, called a vascular guidance tunnel (B). (C) depicts sprout formation and branching with ECM degradation at
by carving out a path for migration, called a vascular guidance tunnel (B). (C) depicts sprout formation and branching with ECM degradation at  , a density not typically conducive to branching, suggesting that high matrix heterogeneity (D) created by tip cell degradation may be a mechanism for branching (Video S3). (E) VEGF gradient profile (pg) shows strong gradient along leading edges of sprout. Snapshots at 14 hours.
, a density not typically conducive to branching, suggesting that high matrix heterogeneity (D) created by tip cell degradation may be a mechanism for branching (Video S3). (E) VEGF gradient profile (pg) shows strong gradient along leading edges of sprout. Snapshots at 14 hours.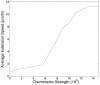
 , chemotactic cues are not strong enough relative to the energies associated with adhesion and growth to induce motility. Above
, chemotactic cues are not strong enough relative to the energies associated with adhesion and growth to induce motility. Above  , chemotactic incentives are so strong relative to adhesion and growth that the cells dissociate.
, chemotactic incentives are so strong relative to adhesion and growth that the cells dissociate.References
-
- Czirok A, Zamir EA, Filla MB, Little CD, Rongish BJ. Extracellular matrix macroassembly dynamics in early vertebrate embryos. Curr Top Dev Biol. 2006;73:237–258. - PubMed
-
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:103–1037. - PubMed
-
- Davis GE, Senger DR. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. - PubMed
-
- Rupp PA, Little CD. Integrins in vascular development. Circ Res. 2001;89:566–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

