Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2)
- PMID: 19629710
- PMCID: PMC2758125
- DOI: 10.1208/s12248-009-9132-1
Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2)
Abstract
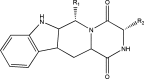
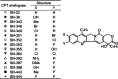
Breast cancer resistance protein (ABCG2), the newest ABC transporter, was discovered independently by three groups in the late 1990s. ABCG2 is widely distributed in the body with expression in the brain, intestine, and liver, among others. ABCG2 plays an important role by effluxing drugs at the blood-brain, blood-testis, and maternal-fetal barriers and in the efflux of xenobiotics at the small intestine and kidney proximal tubule brush border and liver canalicular membranes. ABCG2 transports a wide variety of substrates including HMG-CoA reductase inhibitors, antibiotics, and many anticancer agents and is one contributor to multidrug resistance in cancer cells. Quantitative structure-activity relationship (QSAR) models and structure-activity relationships (SARs) are often employed to predict ABCG2 substrates and inhibitors prior to in vitro and in vivo studies. QSAR models correlate in vivo biological activity to physicochemical properties of compounds while SARs attempt to explain chemical moieties or structural features that contribute to or are detrimental to the biological activity. Most ABCG2 datasets available for in silico modeling are comprised of congeneric series of compounds; the results from one series usually cannot be applied to another series of compounds. This review will focus on in silico models in the literature used for the prediction of ABCG2 substrates and inhibitors.
Figures
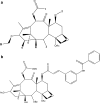
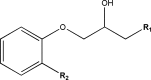
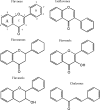
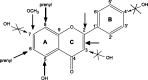
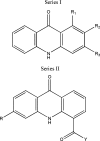
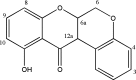
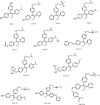
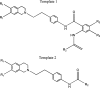
References
-
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–9. - PubMed
-
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. - PubMed
-
- Robey R, Polgar O, Deeken J, To KK, Bates SE. Breast cancer resistance protein. In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug disposition. Hoboken: Wiley; 2007. pp. 319–358.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

