A sequential indium-mediated aldehyde allylation/palladium-catalyzed cross-coupling reaction in the synthesis of 2-deoxy-beta-C-aryl glycosides
- PMID: 19630430
- PMCID: PMC2751794
- DOI: 10.1021/ol901353f
A sequential indium-mediated aldehyde allylation/palladium-catalyzed cross-coupling reaction in the synthesis of 2-deoxy-beta-C-aryl glycosides
Abstract
Indium-mediated allylation of aldehydes with 2-chloro-3-iodopropene, followed by a palladium-catalyzed cross-coupling reaction with triarylindium reagents or arylboronic acids, leads to aryl-substituted homoallylic alcohols in good to excellent yields and diastereoselectivities. The products obtained from reactions conducted with d-glyceraldehyde acetonide can be transformed into 2-deoxy-beta-C-aryl ribofuranosides in high overall yields. Similarly, 2-deoxy-beta-C-aryl allopyranosides may be prepared efficiently from 2,4-O-benzylidene erythrose.
Figures
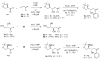
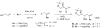
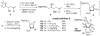
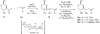
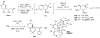
References
-
-
Hansen MR, Hurley LH. Acc Chem Res. 1996;29:249., and references therein.
-
-
- Hacksell U, Daves GD. Prog Med Chem. 1985;22:1. - PubMed
-
- Jaramillo C, Knapp S. Synthesis. 1994;1:1.
- Levy DE, Tang C. The Chemistry of C-glycosides. Elsevier Science; Tarrytown, NY: 1995.
- Postema MHD. In: C-Glycoside Synthesis. Rees CW, editor. CRC Press; Boca Raton, FL: 1995.
- Nicotra F. Top Corr Chem. 1997;187:55.
- Du Y, Linhardt RJ. Tetrahedron. 1998;54:9913.
-
- Matsumoto T, Katsuki M, Suzuki K. Tetrahedron Lett. 1988;29:6935.
- Matsumoto T, Katsuki M, Jona H, Suzuki K. Tetrahedron Lett. 1989;30:6185.
- Matsumoto T, Hosoya T, Suzuki K. Tetrahedron Lett. 1990;31:4629.
- Matsumoto T, Katsuki M, Jona H, Suzuki K. J Am Chem Soc. 1991;113:6982.
-
- Sharma GVM, Raman KK, Sreenivas P, Radha Krishna P, Chorghade MS. Tetrahedron: Asym. 2002;13:687.
- Hosoya T, Takashiro E, Matsumoto T, Suzuki K. J Am Chem Soc. 1994;116:1004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

