Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers
- PMID: 19630438
- PMCID: PMC2774806
- DOI: 10.1021/bi9007879
Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers
Abstract
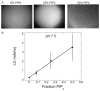
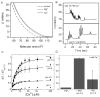
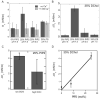
Biological membrane function, in part, depends upon the local regulation of lipid composition. The spatial heterogeneity of membrane lipids has been extensively explored in the context of cholesterol and phospholipid acyl-chain-dependent domain formation, but the effects of lipid head groups and soluble factors in lateral lipid organization are less clear. In this contribution, the effects of divalent calcium ions on domain formation in monolayers containing phosphatidylinositol 4,5-bisphosphate (PIP2), a polyanionic, multifunctional lipid of the cytosolic leaflet of the plasma bilayer, are reported. In binary monolayers of PIP2 mixed with zwitterionic lipids, calcium induced a rapid, PIP2-dependent surface pressure drop, with the concomitant formation of laterally segregated, PIP2-rich domains. The effect was dependent upon head-group multivalency, because lowered pH suppressed the surface-pressure effect and domain formation. In accordance with previous observations, inclusion of cholesterol in lipid mixtures induced coexistence of two liquid phases. Phase separation strongly segregated PIP2 to the cholesterol-poor phase, suggesting a role for cholesterol-dependent lipid demixing in regulating PIP2 localization and local concentration. Similar to binary mixtures, subphase calcium induced contraction of ternary cholesterol-containing monolayers; however, in these mixtures, calcium induced an unexpected, PIP2- and multivalency-dependent decrease in the miscibility phase transition surface pressure, resulting in rapid dissolution of the domains. This result emphasizes the likely critical role of subphase factors and lipid head-group specificity in the formation and stability of cholesterol-dependent domains in cellular plasma membranes.
Figures



References
-
- Esposti MD. Lipids, cardiolipin and apoptosis: a greasy license to kill. Cell Death Differ. 2002;9:234–236. - PubMed
-
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. - PubMed
-
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. - PubMed
-
- Eling TE, Glasgow WC. Cellular proliferation and lipid metabolism: importance of lipoxygenases in modulating epidermal growth factor-dependent mitogenesis. Cancer Metastasis Rev. 1994;13:397–410. - PubMed
-
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

