Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival
- PMID: 19630522
- PMCID: PMC2746923
- DOI: 10.1667/RR1708.1
Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival
Abstract
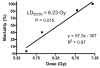
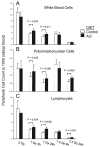
Abstract Dietary antioxidants have radioprotective effects after gamma-radiation exposure that limit hematopoietic cell depletion and improve animal survival. The purpose of this study was to determine whether a dietary supplement consisting of l-selenomethionine, vitamin C, vitamin E succinate, alpha-lipoic acid and N-acetyl cysteine could improve survival of mice after proton total-body irradiation (TBI). Antioxidants significantly increased 30-day survival of mice only when given after irradiation at a dose less than the calculated LD(50/30); for these data, the dose-modifying factor (DMF) was 1.6. Pretreatment of animals with antioxidants resulted in significantly higher serum total white blood cell, polymorphonuclear cell and lymphocyte cell counts at 4 h after 1 Gy but not 7.2 Gy proton TBI. Antioxidants significantly modulated plasma levels of the hematopoietic cytokines Flt-3L and TGFbeta1 and increased bone marrow cell counts and spleen mass after TBI. Maintenance of the antioxidant diet resulted in improved recovery of peripheral leukocytes and platelets after sublethal and potentially lethal TBI. Taken together, oral supplementation with antioxidants appears to be an effective approach for radioprotection of hematopoietic cells and improvement of animal survival after proton TBI.
Figures


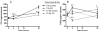
Similar articles
-
Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation.Radiat Res. 2008 Apr;169(4):384-96. doi: 10.1667/RR1204.1. Radiat Res. 2008. PMID: 18363433 Free PMC article.
-
Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality.Radiat Res. 2010 Apr;173(4):462-8. doi: 10.1667/RR1716.1. Radiat Res. 2010. PMID: 20334518 Free PMC article.
-
Whole body proton irradiation causes acute damage to bone marrow hematopoietic progenitor and stem cells in mice.Int J Radiat Biol. 2017 Dec;93(12):1312-1320. doi: 10.1080/09553002.2017.1356941. Epub 2017 Aug 7. Int J Radiat Biol. 2017. PMID: 28782442 Free PMC article.
-
FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits.Blood. 1998 Aug 1;92(3):765-9. Blood. 1998. PMID: 9680342
-
Antioxidant dietary supplementation in mice exposed to proton radiation attenuates expression of programmed cell death-associated genes.Radiat Res. 2011 May;175(5):650-6. doi: 10.1667/RR2330.1. Epub 2011 Mar 28. Radiat Res. 2011. PMID: 21443425 Free PMC article.
Cited by
-
Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets.Int J Radiat Oncol Biol Phys. 2014 Mar 15;88(4):940-6. doi: 10.1016/j.ijrobp.2013.12.001. Epub 2014 Feb 1. Int J Radiat Oncol Biol Phys. 2014. PMID: 24495588 Free PMC article.
-
Changes in patient peripheral blood cell microRNAs after total body irradiation during hematopoietic stem cell transplantation.Ann Transl Med. 2022 Aug;10(16):857. doi: 10.21037/atm-22-3411. Ann Transl Med. 2022. PMID: 36110996 Free PMC article.
-
Acute Hematological Effects in Mice Exposed to the Expected Doses, Dose-rates, and Energies of Solar Particle Event-like Proton Radiation.Life Sci Space Res (Amst). 2014 Jul 1;2:86-91. doi: 10.1016/j.lssr.2014.01.003. Life Sci Space Res (Amst). 2014. PMID: 25202654 Free PMC article.
-
Leukocyte activity is altered in a ground based murine model of microgravity and proton radiation exposure.PLoS One. 2013 Aug 14;8(8):e71757. doi: 10.1371/journal.pone.0071757. eCollection 2013. PLoS One. 2013. PMID: 23977138 Free PMC article.
-
Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss.Transl Res. 2011 Jul;158(1):38-53. doi: 10.1016/j.trsl.2011.02.006. Epub 2011 Mar 21. Transl Res. 2011. PMID: 21708355 Free PMC article.
References
-
- Lundkvist J, Ekman M, Ericsson SR, Jonsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44:850–861. - PubMed
-
- Olsen DR, Bruland OS, Frykholm G, Norderhaug IN. Proton therapy—a systematic review of clinical effectiveness. Radiother. Oncol. 2007;83:123–132. - PubMed
-
- Tsujii H, Tsuji H, Inada T, Maruhashi A, Hayakawa Y, Takada Y, Tada J, Fukumoto S, Tatuzaki H. Clinical results of fractionated proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 1993;25:49–60. - PubMed
-
- Vargas C, Fryer A, Mahajan C, Indelicato D, Horne D, Chellini A, McKenzie C, Lawlor P, Henderson R, Keole S. Dose–volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:744–751. - PubMed
-
- Zietman AL. The Titanic and the iceberg: prostate proton therapy and health care economics. J. Clin. Oncol. 2007;25:3565–3566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

