DOMINO-AD protocol: donepezil and memantine in moderate to severe Alzheimer's disease - a multicentre RCT
- PMID: 19630974
- PMCID: PMC2723100
- DOI: 10.1186/1745-6215-10-57
DOMINO-AD protocol: donepezil and memantine in moderate to severe Alzheimer's disease - a multicentre RCT
Abstract
Background: Alzheimer's disease (AD) is the commonest cause of dementia. Cholinesterase inhibitors, such as donepezil, are the drug class with the best evidence of efficacy, licensed for mild to moderate AD, while the glutamate antagonist memantine has been widely prescribed, often in the later stages of AD. Memantine is licensed for moderate to severe dementia in AD but is not recommended by the England and Wales National Institute for Health and Clinical Excellence. However, there is little evidence to guide clinicians as to what to prescribe as AD advances; in particular, what to do as the condition progresses from moderate to severe. Options include continuing cholinesterase inhibitors irrespective of decline, adding memantine to cholinesterase inhibitors, or prescribing memantine instead of cholinesterase inhibitors. The aim of this trial is to establish the most effective drug option for people with AD who are progressing from moderate to severe dementia despite treatment with donepezil.
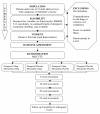
Method: DOMINO-AD is a pragmatic, 15 centre, double-blind, randomized, placebo controlled trial. Patients with AD, currently living at home, receiving donepezil 10 mg daily, and with Standardized Mini-Mental State Examination (SMMSE) scores between 5 and 13 are being recruited. Each is randomized to one of four treatment options: continuation of donepezil with memantine placebo added; switch to memantine with donepezil placebo added; donepezil and memantine together; or donepezil placebo with memantine placebo. 800 participants are being recruited and treatment continues for one year. Primary outcome measures are cognition (SMMSE) and activities of daily living (Bristol Activities of Daily Living Scale). Secondary outcomes are non-cognitive dementia symptoms (Neuropsychiatric Inventory), health related quality of life (EQ-5D and DEMQOL-proxy), carer burden (General Health Questionnaire-12), cost effectiveness (using Client Service Receipt Inventory) and institutionalization. These outcomes are assessed at baseline, 6, 18, 30 and 52 weeks. All participants will be subsequently followed for 3 years by telephone interview to record institutionalization.
Discussion: There is considerable debate about the clinical and cost effectiveness of anti-dementia drugs. DOMINO-AD seeks to provide clear evidence on the best treatment strategies for those managing patients at a particularly important clinical transition point.
Trial registration: Current controlled trials ISRCTN49545035.
References
-
- Areosa SA, Sheriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005. p. CD003154. - PubMed
-
- Rogers SL, Farlow MR, Doody RS, et al. A 24-week double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;44:2308–2314. - PubMed
-
- Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharm. 1998;1:55–65.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical