The novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), inhibits nicotine-evoked [(3)H]norepinephrine overflow from rat hippocampal slices
- PMID: 19631612
- PMCID: PMC3090002
- DOI: 10.1016/j.bcp.2009.07.010
The novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), inhibits nicotine-evoked [(3)H]norepinephrine overflow from rat hippocampal slices
Abstract
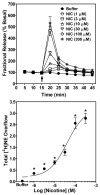
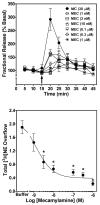
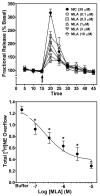
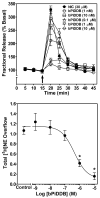
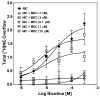
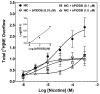
Smoking is a significant health concern and strongly correlated with clinical depression. Depression is associated with decreased extracellular NE concentrations in brain. Smokers may be self-medicating and alleviating their depression through nicotine stimulated norepinephrine (NE) release. Several antidepressants inhibit NE transporter (NET) function, thereby augmenting extracellular NE concentrations. Antidepressants, such as bupropion, also inhibit nicotinic receptor (nAChR) function. The current study determined if a recently discovered novel nAChR antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), inhibits nicotine-evoked NE release from superfused rat hippocampal slices. Previous studies determined that bPiDDB potently (IC(50)=2 nM) inhibits nicotine-evoked striatal [(3)H]dopamine (DA) release in vitro, nicotine-evoked DA release in nucleus accumbens in vivo, and nicotine self-administration in rats. In the current study, nicotine stimulated [(3)H]NE release from rat hippocampal slices (EC(50)=50 microM). bPiDDB inhibited (IC(50)=430 nM; I(max)=90%) [(3)H]NE release evoked by 30 microM nicotine. For comparison, the nonselective nAChR antagonist, mecamylamine, and the alpha7 antagonist, methyllycaconitine, also inhibited nicotine-evoked [(3)H]NE release (IC(50)=31 and 275 nM, respectively; I(max)=91% and 72%, respectively). Inhibition by bPiDDB and mecamylamine was not overcome by increasing nicotine concentrations; Schild regression slope was different from unity, consistent with allosteric inhibition. Thus, bPiDDB was 200-fold more potent inhibiting nAChRs mediating nicotine-evoked [(3)H]DA release from striatum than those mediating nicotine-evoked [(3)H]NE release from hippocampus.
Figures
Similar articles
-
Bupropion inhibits nicotine-evoked [(3)H]overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine.J Pharmacol Exp Ther. 2002 Sep;302(3):1113-22. doi: 10.1124/jpet.102.033852. J Pharmacol Exp Ther. 2002. PMID: 12183670
-
N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity.J Pharmacol Exp Ther. 2008 Aug;326(2):563-76. doi: 10.1124/jpet.108.136630. Epub 2008 May 6. J Pharmacol Exp Ther. 2008. PMID: 18460644 Free PMC article.
-
bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse.Br J Pharmacol. 2011 May;163(2):346-57. doi: 10.1111/j.1476-5381.2011.01220.x. Br J Pharmacol. 2011. PMID: 21232049 Free PMC article.
-
Region-specific effects of N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide on nicotine-induced increase in extracellular dopamine in vivo.Br J Pharmacol. 2008 Feb;153(4):792-804. doi: 10.1038/sj.bjp.0707612. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059317 Free PMC article.
-
Targeting nicotinic receptor antagonists as novel pharmacotherapies for tobacco dependence and relapse.Neuropsychopharmacology. 2009 Jan;34(1):244-6. doi: 10.1038/npp.2008.157. Neuropsychopharmacology. 2009. PMID: 19079069 Free PMC article. Review. No abstract available.
Cited by
-
Bis-azaaromatic quaternary ammonium salts as ligands for the blood-brain barrier choline transporter.Bioorg Med Chem Lett. 2010 Jun 1;20(11):3208-10. doi: 10.1016/j.bmcl.2010.04.098. Epub 2010 Apr 24. Bioorg Med Chem Lett. 2010. PMID: 20462759 Free PMC article.
-
Nicotinic receptor antagonists as treatments for nicotine abuse.Adv Pharmacol. 2014;69:513-51. doi: 10.1016/B978-0-12-420118-7.00013-5. Adv Pharmacol. 2014. PMID: 24484986 Free PMC article. Review.
-
Predictive screening model for potential vector-mediated transport of cationic substrates at the blood-brain barrier choline transporter.Bioorg Med Chem Lett. 2010 Feb 1;20(3):870-7. doi: 10.1016/j.bmcl.2009.12.079. Epub 2009 Dec 28. Bioorg Med Chem Lett. 2010. PMID: 20053562 Free PMC article.
References
-
- Rose JE. Disrupting nicotine reinforcement: from cigarette to brain. Ann NY Acad Sci. 2008;1141:233–56. - PubMed
-
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–9. - PubMed
-
- Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

