VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation
- PMID: 19631637
- PMCID: PMC2871302
- DOI: 10.1016/j.ydbio.2009.07.023
VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation
Abstract
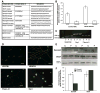
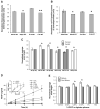
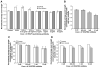
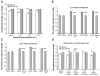
Sympathetic nerve activity regulates blood pressure by altering peripheral vascular resistance. Variations in vascular sympathetic innervation suggest that vascular-derived cues promote selective innervation of particular vessels during development. As axons extend towards peripheral targets, they migrate along arterial networks following gradients of guidance cues. Collective ratios of these gradients may determine whether axons grow towards and innervate vessels or continue past non-innervated vessels towards peripheral targets. Utilizing directed neurite outgrowth in a three-dimensional (3D) co-culture, we observed increased axon growth from superior cervical ganglion explants (SCG) towards innervated compared to non-innervated vessels, mediated in part by vascular endothelial growth factor (VEGF-A) and Semaphorin3A (Sema3A) which both signal via neuropilin-1 (Nrp1). Exogenous VEGF-A, delivered by high-expressing VEGF-A-LacZ vessels or by rhVEGF-A/alginate spheres, increased sympathetic neurite outgrowth while exogenous rhSema3A/Fc decreased neurite outgrowth. VEGF-A expression is similar between the innervated and non-innervated vessels examined. Sema3A expression is higher in non-innervated vessels. Spatial gradients of Sema3A and VEGF-A may promote differential Nrp1 binding. Vessels expressing high levels of Sema3A favor Nrp1-PlexinA1 signaling, producing chemorepulsive cues limiting sympathetic neurite outgrowth and vascular innervation; while low Sema3A expressing vessels favor Nrp1-VEGFR2 signaling providing chemoattractive cues for sympathetic neurite outgrowth and vascular innervation.
Figures



Similar articles
-
NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system.Dev Biol. 2012 Sep 15;369(2):277-85. doi: 10.1016/j.ydbio.2012.06.026. Epub 2012 Jul 10. Dev Biol. 2012. PMID: 22790009 Free PMC article.
-
Neuropilin ligands in vascular and neuronal patterning.Biochem Soc Trans. 2009 Dec;37(Pt 6):1228-32. doi: 10.1042/BST0371228. Biochem Soc Trans. 2009. PMID: 19909252 Free PMC article.
-
Selective requirements for NRP1 ligands during neurovascular patterning.Development. 2007 May;134(10):1833-43. doi: 10.1242/dev.002402. Epub 2007 Apr 11. Development. 2007. PMID: 17428830 Free PMC article.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
-
Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability.Microcirculation. 2014 May;21(4):315-23. doi: 10.1111/micc.12124. Microcirculation. 2014. PMID: 24521511 Free PMC article. Review.
Cited by
-
Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues.Cell Mol Life Sci. 2013 May;70(10):1685-703. doi: 10.1007/s00018-013-1278-4. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475066 Free PMC article. Review.
-
Vascular endothelial growth factor protects post-ganglionic sympathetic neurones from the detrimental effects of hydrogen peroxide by increasing catalase.Acta Physiol (Oxf). 2011 Sep;203(1):271-8. doi: 10.1111/j.1748-1716.2011.02258.x. Epub 2011 Mar 14. Acta Physiol (Oxf). 2011. PMID: 21276205 Free PMC article.
-
Neurovascular proximity in the diaphragm muscle of adult mice.Microcirculation. 2012 May;19(4):306-15. doi: 10.1111/j.1549-8719.2012.00163.x. Microcirculation. 2012. PMID: 22268653 Free PMC article.
-
Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus.Nat Rev Endocrinol. 2019 Apr;15(4):207-225. doi: 10.1038/s41574-019-0165-y. Nat Rev Endocrinol. 2019. PMID: 30733616 Free PMC article. Review.
-
Epigenetically heterogeneous tumor cells direct collective invasion through filopodia-driven fibronectin micropatterning.Sci Adv. 2020 Jul 24;6(30):eaaz6197. doi: 10.1126/sciadv.aaz6197. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32832657 Free PMC article.
References
-
- Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902–4912. - PMC - PubMed
-
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. - PMC - PubMed
-
- Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res. 2008;45:323–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

