Mitogen-activated protein kinase p38alpha and retinal ischemic preconditioning
- PMID: 19631642
- PMCID: PMC2782459
- DOI: 10.1016/j.exer.2009.07.006
Mitogen-activated protein kinase p38alpha and retinal ischemic preconditioning
Abstract
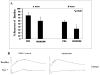
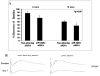
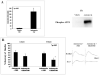
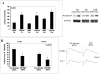
In previous studies, inhibition of mitogen-activated protein kinase (MAP) p38 significantly improved recovery and attenuated apoptosis after retinal ischemia in rats. Yet, ischemic preconditioning (IPC) attenuated the ischemia-induced increase in p38 expression. We hypothesized that p38 was required for induction of ischemic tolerance by IPC. We examined the mechanisms of involvement of p38 in IPC neuroprotection. IPC or ischemia was induced in rat retina in vivo. Recovery after ischemia performed 24h after IPC was assessed functionally (electroretinography) and histologically at 7d after ischemia in the presence or absence of inhibition of p38. We examined the role of p38alpha in the mimicking of IPC produced by opening mitochondrial KATP channels using diazoxide, or stimulation of p38 activation by anisomycin. The importance of adenosine receptors in p38 activation after IPC was assessed using specific blockers of adenosine A1 and A2a receptors. Interfering RNA (siRNA) or SB203580 was used to block p38alpha. Phosphorylated p38 levels were measured. Phosphorylated p38 protein increased with IPC. Interfering RNA (siRNA) to p38alpha prior to IPC, or inhibiting p38 activation with SB203580, with ischemia following 24h later, significantly attenuated the neuroprotective effect of IPC. Anisomycin administered to increase p38 mimicked IPC, an effect blocked by SB203580. IPC-mimicking with diazoxide, an opener of mitochondrial KATP channels, was diminished with p38alpha siRNA. Adenosine receptor blockade did not decrease the elevated levels of phosphorylated p38 after IPC. Specific inhibition of p38alpha suggests that this MAPK is involved in the protective effects of IPC, and that p38 is downstream of mitochondrial KATP channels, but not adenosine receptors, in this neuroprotection.
Figures



References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. - PubMed
-
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. - PubMed
-
- Cross TG, Scheel-Toellner D, Henriquez NK, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

