Identification of c-Src tyrosine kinase substrates in platelet-derived growth factor receptor signaling
- PMID: 19632164
- PMCID: PMC2783305
- DOI: 10.1016/j.molonc.2009.07.001
Identification of c-Src tyrosine kinase substrates in platelet-derived growth factor receptor signaling
Abstract
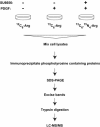
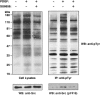
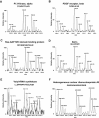
c-Src non-receptor tyrosine kinase is an important component of the platelet-derived growth factor (PDGF) receptor signaling pathway. c-Src has been shown to mediate the mitogenic response to PDGF in fibroblasts. However, the exact components of PDGF receptor signaling pathway mediated by c-Src remain unclear. Here, we used stable isotope labeling with amino acids in cell culture (SILAC) coupled with mass spectrometry to identify Src-family kinase substrates involved in PDGF signaling. Using SILAC, we were able to detect changes in tyrosine phosphorylation patterns of 43 potential c-Src kinase substrates in PDGF receptor signaling. This included 23 known c-Src kinase substrates, of which 16 proteins have known roles in PDGF signaling while the remaining 7 proteins have not previously been implicated in PDGF receptor signaling. Importantly, our analysis also led to identification of 20 novel Src-family kinase substrates, of which 5 proteins were previously reported as PDGF receptor signaling pathway intermediates while the remaining 15 proteins represent novel signaling intermediates in PDGF receptor signaling. In validation experiments, we demonstrated that PDGF indeed induced the phosphorylation of a subset of candidate Src-family kinase substrates - Calpain 2, Eps15 and Trim28 - in a c-Src-dependent fashion.
Figures

Similar articles
-
Platelet-derived growth factor (PDGF)-induced activation of signal transducer and activator of transcription (Stat) 5 is mediated by PDGF beta-receptor and is not dependent on c-src, fyn, jak1 or jak2 kinases.Biochem J. 2000 Feb 1;345 Pt 3(Pt 3):759-66. Biochem J. 2000. PMID: 10642538 Free PMC article.
-
Sphingosine 1-phosphate stimulation of NADPH oxidase activity: relationship with platelet-derived growth factor receptor and c-Src kinase.Biochim Biophys Acta. 2007 Jun;1770(6):872-83. doi: 10.1016/j.bbagen.2007.01.008. Epub 2007 Feb 1. Biochim Biophys Acta. 2007. PMID: 17349748
-
Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF.J Biol Chem. 2002 May 3;277(18):15499-506. doi: 10.1074/jbc.M200427200. Epub 2002 Feb 19. J Biol Chem. 2002. PMID: 11854294
-
Platelet derived growth factor/tyrosine kinase receptor mediated proliferation.Growth Regul. 1993 Sep;3(3):172-9. Growth Regul. 1993. PMID: 8220110 Review.
-
The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation.Curr Mol Med. 2003 Jun;3(4):313-24. doi: 10.2174/1566524033479744. Curr Mol Med. 2003. PMID: 12776987 Review.
Cited by
-
Phosphorylation of KRAB-associated protein 1 (KAP1) at Tyr-449, Tyr-458, and Tyr-517 by nuclear tyrosine kinases inhibits the association of KAP1 and heterochromatin protein 1α (HP1α) with heterochromatin.J Biol Chem. 2013 Jun 14;288(24):17871-83. doi: 10.1074/jbc.M112.437756. Epub 2013 May 4. J Biol Chem. 2013. PMID: 23645696 Free PMC article.
-
Differential phosphoproteomics of fibroblast growth factor signaling: identification of Src family kinase-mediated phosphorylation events.J Proteome Res. 2010 May 7;9(5):2317-28. doi: 10.1021/pr9010475. J Proteome Res. 2010. PMID: 20225815 Free PMC article.
-
Quantitative Phosphoproteomics Reveals a Role for Collapsin Response Mediator Protein 2 in PDGF-Induced Cell Migration.Sci Rep. 2017 Jun 21;7(1):3970. doi: 10.1038/s41598-017-04015-x. Sci Rep. 2017. PMID: 28638064 Free PMC article.
-
Systems-wide analysis of K-Ras, Cdc42, and PAK4 signaling by quantitative phosphoproteomics.Mol Cell Proteomics. 2013 Aug;12(8):2070-80. doi: 10.1074/mcp.M112.027052. Epub 2013 Apr 22. Mol Cell Proteomics. 2013. PMID: 23608596 Free PMC article.
-
An adaptive Src-PDGFRA-Raf axis in rhabdomyosarcoma.Biochem Biophys Res Commun. 2012 Sep 28;426(3):363-8. doi: 10.1016/j.bbrc.2012.08.092. Epub 2012 Aug 30. Biochem Biophys Res Commun. 2012. PMID: 22960170 Free PMC article.
References
-
- Amanchy, R. , Kalume, D.E. , Iwahori, A. , Zhong, J. , Pandey, A. , 2005. Phosphoproteome analysis of HeLa cells using stable isotope labeling with amino acids in cell culture (SILAC). J. Proteome Res.. 4, 1661–1671. - PubMed
-
- Amanchy, R. , Kalume, D.E. , Pandey, A. , 2005. Stable isotope labeling with amino acids in cell culture (SILAC) for studying dynamics of protein abundance and posttranslational modifications. Sci. STKE. 2005, pl2 - PubMed
-
- Auger, K.R. , Serunian, L.A. , Soltoff, S.P. , Libby, P. , Cantley, L.C. , 1989. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 57, 167–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

