The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis
- PMID: 19632185
- PMCID: PMC2774252
- DOI: 10.1016/j.cell.2009.05.022
The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis
Erratum in
- Cell. 2009 Sep 4;138(5):1032
Abstract
Prostate apoptosis response-4 (Par-4) is a proapoptotic protein with intracellular functions in the cytoplasm and nucleus. Unexpectedly, we noted Par-4 protein is spontaneously secreted by normal and cancer cells in culture, and by Par-4 transgenic mice that are resistant to spontaneous tumors. Short exposure to endoplasmic reticulum (ER) stress-inducing agents further increased cellular secretion of Par-4 by a brefeldin A-sensitive pathway. Secretion occurred independently of caspase activation and apoptosis. Interestingly, extracellular Par-4 induced apoptosis by binding to the stress response protein, glucose-regulated protein-78 (GRP78), expressed at the surface of cancer cells. The interaction of extracellular Par-4 and cell surface GRP78 led to apoptosis via ER stress and activation of the FADD/caspase-8/caspase-3 pathway. Moreover, apoptosis inducible by TRAIL, which also exerts cancer cell-specific effects, is dependent on extracellular Par-4 signaling via cell surface GRP78. Thus, Par-4 activates an extrinsic pathway involving cell surface GRP78 receptor for induction of apoptosis.
Figures


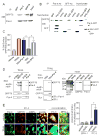
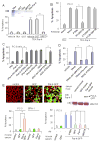
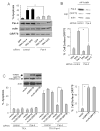

Comment in
-
Cell death: a new Par-4 the TRAIL.Cell. 2009 Jul 23;138(2):220-2. doi: 10.1016/j.cell.2009.07.007. Cell. 2009. PMID: 19632170
Similar articles
-
The Par-4-GRP78 TRAIL, more twists and turns.Cancer Biol Ther. 2009 Nov;8(22):2103-5. doi: 10.4161/cbt.8.22.10140. Epub 2009 Nov 20. Cancer Biol Ther. 2009. PMID: 19823030 Free PMC article.
-
Mechanisms of apoptosis by the tumor suppressor Par-4.J Cell Physiol. 2012 Dec;227(12):3715-21. doi: 10.1002/jcp.24098. J Cell Physiol. 2012. PMID: 22552839 Free PMC article. Review.
-
Role of prostate apoptosis response 4 in translocation of GRP78 from the endoplasmic reticulum to the cell surface of trophoblastic cells.PLoS One. 2013 Nov 25;8(11):e80231. doi: 10.1371/journal.pone.0080231. eCollection 2013. PLoS One. 2013. PMID: 24282526 Free PMC article.
-
Geranylgeranylacetone, an inducer of the 70-kDa heat shock protein (HSP70), elicits unfolded protein response and coordinates cellular fate independently of HSP70.Mol Pharmacol. 2007 Nov;72(5):1337-48. doi: 10.1124/mol.107.039164. Epub 2007 Aug 16. Mol Pharmacol. 2007. PMID: 17702888
-
Cancer-selective apoptotic effects of extracellular and intracellular Par-4.Oncogene. 2010 Jul 8;29(27):3873-80. doi: 10.1038/onc.2010.141. Epub 2010 May 3. Oncogene. 2010. PMID: 20440265 Free PMC article. Review.
Cited by
-
GRP78-targeted nanotherapy against castrate-resistant prostate cancer cells expressing membrane GRP78.Target Oncol. 2013 Dec;8(4):225-30. doi: 10.1007/s11523-012-0234-9. Epub 2012 Oct 23. Target Oncol. 2013. PMID: 23090204
-
4th international conference on tumor progression and therapeutic resistance: meeting report.Cancer Biol Ther. 2015;16(3):363-76. doi: 10.1080/15384047.2015.1004928. Cancer Biol Ther. 2015. PMID: 25782066 Free PMC article.
-
A brief overview about the adipokine: Isthmin-1.Front Cardiovasc Med. 2022 Jul 26;9:939757. doi: 10.3389/fcvm.2022.939757. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35958402 Free PMC article. Review.
-
Turn TRAIL Into Better Anticancer Therapeutic Through TRAIL Fusion Proteins.Cancer Med. 2025 Jan;14(1):e70517. doi: 10.1002/cam4.70517. Cancer Med. 2025. PMID: 39740038 Free PMC article. Review.
-
The long noncoding RNA of RMRP is downregulated by PERK, which induces apoptosis in hepatocellular carcinoma cells.Sci Rep. 2021 Apr 12;11(1):7926. doi: 10.1038/s41598-021-86592-6. Sci Rep. 2021. PMID: 33846370 Free PMC article.
References
-
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. - PubMed
-
- Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. - PubMed
-
- Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001;61:7255–7263. - PubMed
-
- Cheema SK, Mishra SK, Rangnekar VM, Tari AM, Kumar R, Lopez-Berestein G. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem. 2003;278:19995–20005. - PubMed
-
- Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, Linehan WM, Sukhatme VP, Weinstein MH, Rangnekar VM. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene. 1999;18:1205–1208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

