The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli
- PMID: 19632231
- PMCID: PMC2789877
- DOI: 10.1053/j.gastro.2009.07.048
The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli
Abstract
Background & aims: The transient receptor potential (TRP) channel family includes transducers of mechanical and chemical stimuli for visceral sensory neurons. TRP ankyrin 1 (TRPA1) is implicated in inflammatory pain; it interacts with G-protein-coupled receptors, but little is known about its role in the gastrointestinal (GI) tract. Sensory information from the GI tract is conducted via 5 afferent subtypes along 3 pathways.
Methods: Nodose and dorsal root ganglia whose neurons innnervate 3 different regions of the GI tract were analyzed from wild-type and TRPA1(-/-) mice using quantitative reverse-transcription polymerase chain reaction, retrograde labeling, and in situ hybridization. Distal colon sections were analyzed by immunohistochemistry. In vitro electrophysiology and pharmacology studies were performed, and colorectal distension and visceromotor responses were measured. Colitis was induced by administration of trinitrobenzene sulphonic acid.
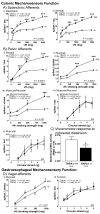
Results: TRPA1 is required for normal mechano- and chemosensory function in specific subsets of vagal, splanchnic, and pelvic afferents. The behavioral responses to noxious colonic distension were substantially reduced in TRPA1(-/-) mice. TRPA1 agonists caused mechanical hypersensitivity, which increased in mice with colitis. Colonic afferents were activated by bradykinin and capsaicin, which mimic effects of tissue damage; wild-type and TRPA1(-/-) mice had similar direct responses to these 2 stimuli. After activation by bradykinin, wild-type afferents had increased mechanosensitivity, whereas, after capsaicin exposure, mechanosensitivity was reduced: these changes were absent in TRPA1(-/-) mice. No interaction between protease-activated receptor-2 and TRPA1 was evident.
Conclusions: These findings demonstrate a previously unrecognized role for TRPA1 in normal and inflamed mechanosensory function and nociception within the viscera.
Conflict of interest statement
None of the authors has any conflict of interest to declare
Figures





References
-
- Lembo T, et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–96. - PubMed
-
- Azpiroz F, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62–88. - PubMed
-
- Barbara G, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. - PubMed
-
- Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

