Mass spectrometry identifies multiple organophosphorylated sites on tubulin
- PMID: 19632257
- PMCID: PMC2753752
- DOI: 10.1016/j.taap.2009.07.020
Mass spectrometry identifies multiple organophosphorylated sites on tubulin
Abstract
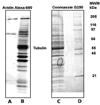
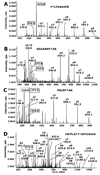
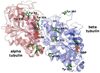
Acute toxicity of organophosphorus poisons (OP) is explained by inhibition of acetylcholinesterase in nerve synapses. Low-dose effects are hypothesized to result from modification of other proteins, whose identity is not yet established. The goal of the present work was to obtain information that would make it possible to identify tubulin as a target of OP exposure. Tubulin was selected for study because live mice injected with a nontoxic dose of a biotinylated organophosphorus agent appeared to have OP-labeled tubulin in brain as determined by binding to avidin beads and mass spectrometry. The experiments with live mice were not conclusive because binding to avidin beads could be nonspecific. To be convincing, it is necessary to find and characterize the OP-labeled tubulin peptide. The search for OP-labeled tubulin peptides was begun by identifying residues capable of making a covalent bond with OP. Pure bovine tubulin (0.012 mM) was treated with 0.01-0.5 mM chlorpyrifos oxon for 24 h at 37 degrees C in pH 8.3 buffer. The identity of labeled amino acids and percent labeling was determined by mass spectrometry. Chlorpyrifos oxon bound covalently to tyrosines 83, 103, 108, 161, 224, 262, 272, 357, and 399 in bovine alpha tubulin, and to tyrosines 50, 51, 59, 106, 159, 281, 310, and 340 in bovine beta tubulin. The most reactive were tyrosine 83 in alpha and tyrosine 281 in beta tubulin. In the presence of 1 mM GTP, percent labeling increased 2-fold. Based on the crystal structure of the tubulin heterodimer (PDB 1jff) tyrosines 83 and 281 are well exposed to solvent. In conclusion seventeen tyrosines in tubulin have the potential to covalently bind chlorpyrifos oxon. These results will be useful when searching for OP-labeled tubulin in live animals.
Figures
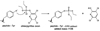



Similar articles
-
Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents.Toxicol Sci. 2010 May;115(1):183-93. doi: 10.1093/toxsci/kfq032. Epub 2010 Feb 8. Toxicol Sci. 2010. PMID: 20142434 Free PMC article.
-
Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity.Toxicol Appl Pharmacol. 2009 Oct 15;240(2):143-8. doi: 10.1016/j.taap.2009.07.015. Epub 2009 Jul 22. Toxicol Appl Pharmacol. 2009. PMID: 19631231 Free PMC article.
-
Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents.Chem Biol Interact. 2008 Sep 25;175(1-3):180-6. doi: 10.1016/j.cbi.2008.04.013. Epub 2008 Apr 22. Chem Biol Interact. 2008. PMID: 18502412 Free PMC article.
-
Review of tyrosine and lysine as new motifs for organophosphate binding to proteins that have no active site serine.Chem Biol Interact. 2010 Sep 6;187(1-3):344-8. doi: 10.1016/j.cbi.2010.03.002. Epub 2010 Mar 6. Chem Biol Interact. 2010. PMID: 20211158 Free PMC article. Review.
-
Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature.J Toxicol Environ Health. 1995 Feb;44(2):135-65. doi: 10.1080/15287399509531952. J Toxicol Environ Health. 1995. PMID: 7531775 Review.
Cited by
-
Cresyl saligenin phosphate, an organophosphorus toxicant, makes covalent adducts with histidine, lysine, and tyrosine residues of human serum albumin.Chem Res Toxicol. 2012 Aug 20;25(8):1752-61. doi: 10.1021/tx300215g. Epub 2012 Jul 26. Chem Res Toxicol. 2012. PMID: 22793878 Free PMC article.
-
The inhibition, reactivation and mechanism of VX-, sarin-, fluoro-VX and fluoro-sarin surrogates following their interaction with HuAChE and HuBuChE.Chem Biol Interact. 2018 Aug 1;291:220-227. doi: 10.1016/j.cbi.2018.06.019. Epub 2018 Jun 18. Chem Biol Interact. 2018. PMID: 29920286 Free PMC article.
-
Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents.Toxicol Sci. 2010 May;115(1):183-93. doi: 10.1093/toxsci/kfq032. Epub 2010 Feb 8. Toxicol Sci. 2010. PMID: 20142434 Free PMC article.
-
Preparation and characterization of diethoxy- and monoethoxy phosphylated ('aged') serine haptens and use in the production of monoclonal antibodies.Chem Biol Interact. 2014 Nov 5;223:134-40. doi: 10.1016/j.cbi.2014.09.010. Epub 2014 Sep 26. Chem Biol Interact. 2014. PMID: 25261769 Free PMC article.
-
Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: characteristic mass fragments and a new binding motif for organophosphates.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 May 15;878(17-18):1297-311. doi: 10.1016/j.jchromb.2009.07.026. Epub 2009 Jul 24. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 19762289 Free PMC article.
References
-
- Abou-Donia MB. Involvement of cytoskeletal proteins in the mechanisms of organophosphorus ester-induced delayed neurotoxicity. Clin Exp Pharmacol Physiol. 1995;22:358–359. - PubMed
-
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–497. - PubMed
-
- Adams TK, Capacio BR, Smith JR, Whalley CE, Korte WD. The application of the fluoride reactivation process to the detection of sarin and soman nerve agent exposures in biological samples. Drug Chem. Toxicol. 2004;27:77–91. - PubMed
-
- Azaroff LS. Biomarkers of exposure to organophosphorous insecticides among farmers' families in rural El Salvador: factors associated with exposure. Environ Res. 1999;80:138–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

