Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation
- PMID: 19632626
- PMCID: PMC3038671
- DOI: 10.1016/j.hrthm.2009.04.015
Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation
Abstract
Background: Mutations in several ion channel genes have been reported to cause rare cases of familial atrial fibrillation (AF).
Objective: The purpose of this study was to determine the genetic basis for AF in a family with autosomal dominant AF.
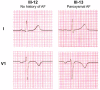
Methods: Family members were evaluated by 12-lead ECG, echocardiogram, signal-averaged P-wave analysis, and laboratory studies. Fourteen family members in AF-324 were studied. Six individuals had AF, with a mean age at onset of 32 years (range 16-59 years).
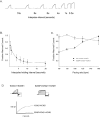
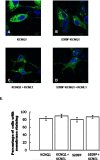
Results: Compared with unaffected family members, those with AF had a longer mean QRS duration (100 vs 86 ms, P = .015) but no difference in the corrected QT interval (423 +/- 15 ms vs 421 +/- 21 ms). The known loci for AF and other cardiovascular diseases were evaluated. Evidence of linkage was obtained with marker D11S4088 located within KCNQ1, and a highly conserved serine in the third transmembrane region was found to be mutated to a proline (S209P). Compared to the wild-type channel, the S209P mutant activates more rapidly, deactivates more slowly, and has a hyperpolarizing shift in the voltage activation curve. A fraction of the mutant channels are constitutively open at all voltages, resulting in a net increase in I(Ks) current.
Conclusion: We identified a family with lone AF due to a mutation in the highly conserved S3 domain of KCNQ1, a region of the channel not previously implicated in the pathogenesis of AF.
Figures


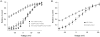
Comment in
-
Kv7.1 in atrial fibrillation.Heart Rhythm. 2009 Aug;6(8):1154-5. doi: 10.1016/j.hrthm.2009.05.004. Epub 2009 May 8. Heart Rhythm. 2009. PMID: 19560405 No abstract available.
References
-
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. - PubMed
-
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. - PubMed
-
- Fox CS, Parise H, D'Agostino RB, Sr., et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851–2855. - PubMed
-
- Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118(2):179–184. - PubMed
-
- Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336(13):905–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

