Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial
- PMID: 19632716
- PMCID: PMC4407808
- DOI: 10.1016/S0140-6736(09)60737-6
Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial
Abstract
Background: Results from phase II studies in patients with stage IIIA non-small-cell lung cancer with ipsilateral mediastinal nodal metastases (N2) have shown the feasibility of resection after concurrent chemotherapy and radiotherapy with promising rates of survival. We therefore did this phase III trial to compare concurrent chemotherapy and radiotherapy followed by resection with standard concurrent chemotherapy and definitive radiotherapy without resection.
Methods: Patients with stage T1-3pN2M0 non-small-cell lung cancer were randomly assigned in a 1:1 ratio to concurrent induction chemotherapy (two cycles of cisplatin [50 mg/m(2) on days 1, 8, 29, and 36] and etoposide [50 mg/m(2) on days 1-5 and 29-33]) plus radiotherapy (45 Gy) in multiple academic and community hospitals. If no progression, patients in group 1 underwent resection and those in group 2 continued radiotherapy uninterrupted up to 61 Gy. Two additional cycles of cisplatin and etoposide were given in both groups. The primary endpoint was overall survival (OS). Analysis was by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00002550.
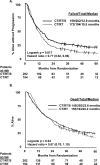
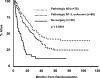
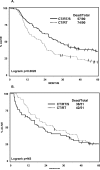
Findings: 202 patients (median age 59 years, range 31-77) were assigned to group 1 and 194 (61 years, 32-78) to group 2. Median OS was 23.6 months (IQR 9.0-not reached) in group 1 versus 22.2 months (9.4-52.7) in group 2 (hazard ratio [HR] 0.87 [0.70-1.10]; p=0.24). Number of patients alive at 5 years was 37 (point estimate 27%) in group 1 and 24 (point estimate 20%) in group 2 (odds ratio 0.63 [0.36-1.10]; p=0.10). With N0 status at thoracotomy, the median OS was 34.4 months (IQR 15.7-not reached; 19 [point estimate 41%] patients alive at 5 years). Progression-free survival (PFS) was better in group 1 than in group 2, median 12.8 months (5.3-42.2) vs 10.5 months (4.8-20.6), HR 0.77 [0.62-0.96]; p=0.017); the number of patients without disease progression at 5 years was 32 (point estimate 22%) versus 13 (point estimate 11%), respectively. Neutropenia and oesophagitis were the main grade 3 or 4 toxicities associated with chemotherapy plus radiotherapy in group 1 (77 [38%] and 20 [10%], respectively) and group 2 (80 [41%] and 44 [23%], respectively). In group 1, 16 (8%) deaths were treatment related versus four (2%) in group 2. In an exploratory analysis, OS was improved for patients who underwent lobectomy, but not pneumonectomy, versus chemotherapy plus radiotherapy.
Interpretation: Chemotherapy plus radiotherapy with or without resection (preferably lobectomy) are options for patients with stage IIIA(N2) non-small-cell lung cancer.
Funding: National Cancer Institute, Canadian Cancer Society, and National Cancer Institute of Canada.
Figures
Comment in
-
Surgery in stage III non-small-cell lung cancer.Lancet. 2009 Aug 1;374(9687):359-60. doi: 10.1016/S0140-6736(09)61026-6. Epub 2009 Jul 24. Lancet. 2009. PMID: 19632715 No abstract available.
-
Surgery: Future directions in multimodality therapy for NSCLC.Nat Rev Clin Oncol. 2010 Jan;7(1):10-2. doi: 10.1038/nrclinonc.2009.174. Nat Rev Clin Oncol. 2010. PMID: 20029443
-
Current readings: management of N2 disease for lung cancer.Semin Thorac Cardiovasc Surg. 2014 Spring;26(1):67-70. doi: 10.1053/j.semtcvs.2014.02.008. Epub 2014 Feb 27. Semin Thorac Cardiovasc Surg. 2014. PMID: 24952759
References
-
- Mountain CF. Prognostic implications of the International Staging System for lung cancer. Semin Oncol. 1988;3:236–245. - PubMed
-
- Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Amer. 1987;67:1037–49. - PubMed
-
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. - PubMed
-
- Johnson DH, Einhorn LH, Bartolucci A, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non-small cell lung cancer. Annals Int Med. 1990;113:33–38. - PubMed
-
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell-lung cancer. N Engl J Med. 1990;323:940–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA077202/CA/NCI NIH HHS/United States
- CA03927/CA/NCI NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- CA32115/CA/NCI NIH HHS/United States
- CA49957/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA049957/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
- U10 CA032115/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- CA21661/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA037422/CA/NCI NIH HHS/United States
- CA37422/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

