Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors
- PMID: 19632986
- PMCID: PMC2788857
- DOI: 10.1074/jbc.M109.034231
Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors
Abstract
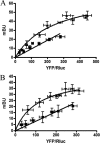
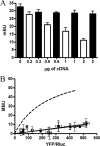
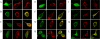
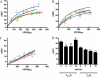
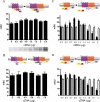
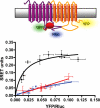
The Ca(2+)-binding protein calmodulin (CaM) has been shown to bind directly to cytoplasmic domains of some G protein-coupled receptors, including the dopamine D(2) receptor. CaM binds to the N-terminal portion of the long third intracellular loop of the D(2) receptor, within an Arg-rich epitope that is also involved in the binding to G(i/o) proteins and to the adenosine A(2A) receptor, with the formation of A(2A)-D(2) receptor heteromers. In the present work, by using proteomics and bioluminescence resonance energy transfer (BRET) techniques, we provide evidence for the binding of CaM to the A(2A) receptor. By using BRET and sequential resonance energy transfer techniques, evidence was obtained for CaM-A(2A)-D(2) receptor oligomerization. BRET competition experiments indicated that, in the A(2A)-D(2) receptor heteromer, CaM binds preferentially to a proximal C terminus epitope of the A(2A) receptor. Furthermore, Ca(2+) was found to induce conformational changes in the CaM-A(2A)-D(2) receptor oligomer and to selectively modulate A(2A) and D(2) receptor-mediated MAPK signaling in the A(2A)-D(2) receptor heteromer. These results may have implications for basal ganglia disorders, since A(2A)-D(2) receptor heteromers are being considered as a target for anti-parkinsonian agents.
Figures
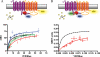
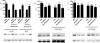
References
-
- Agnati L. F., Ferré S., Lluis C., Franco R., Fuxe K. (2003) Pharmacol. Rev. 55, 509–550 - PubMed
-
- Pin J. P., Neubig R., Bouvier M., Devi L., Filizola M., Javitch J. A., Lohse M. J., Milligan G., Palczewski K., Parmentier M., Spedding M. (2007) Pharmacol. Rev. 59, 5–13 - PubMed
-
- Lee S. P., O'Dowd B. F., George S. R. (2003) Life Sci. 74, 173–180 - PubMed
-
- Prinster S. C., Holmqvist T. G., Hall R. A. (2006) J. Pharmacol. Exp. Ther. 318, 974–981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

