Dual acylation of PDE2A splice variant 3: targeting to synaptic membranes
- PMID: 19632989
- PMCID: PMC2757980
- DOI: 10.1074/jbc.M109.017194
Dual acylation of PDE2A splice variant 3: targeting to synaptic membranes
Abstract
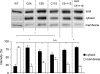
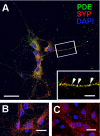
The cGMP-stimulated PDE2A hydrolyzes both cyclic nucleotides, cGMP and cAMP. Three splice variants have been cloned from several species. Whereas PDE2A1 is soluble, PDE2A2 and PDE2A3 are membrane-bound enzymes of rat and bovine origin, respectively. To date it is unclear whether one species expresses all three variants. The splice variants only differ in their N termini, which likely determine the subcellular localization. However, the mechanism for membrane attachment remains unknown. Here, we show that myristoylation underlies membrane targeting of PDE2A3. The myristoylated enzyme was bound to plasma membranes, whereas mutation of the myristoyl recipient Gly2 prevented incorporation of [3H]myristate and turned PDE2A3 completely soluble. Additionally, Cys5 and to a minor extent Cys11 are required for targeting of PDE2A3. Substitution of the putatively palmitoylated cysteines partially solubilized the enzyme and led to an accumulation in the endoplasmic reticulum/Golgi compartment, as shown by fluorescence microscopy in HEK 293 and PC12 cells. In vivo, PDE2A is expressed in many tissues. By using newly generated antibodies selectively detecting the splice variants PDE2A3 or PDE2A1, respectively, we demonstrate on the protein level PDE2A3 expression in mouse brain where it is entirely membrane-associated and a widespread expression of soluble PDE2A1 in mouse tissues. We show that PDE2A localizes to synaptosomal membranes and in primary cultures of hippocampal neurons partially overlaps with the presynaptic marker synaptophysin as demonstrated by immunofluorescence. In sum, these results demonstrate dual acylation as mechanism targeting neuronal PDE2A3 to synapses thereby ensuring local control of cyclic nucleotides.
Figures





References
-
- Bender A. T., Beavo J. A. (2006) Pharmacol. Rev. 58, 488–520 - PubMed
-
- Martins T. J., Mumby M. C., Beavo J. A. (1982) J. Biol. Chem. 257, 1973–1979 - PubMed
-
- Beavo J. A., Hardman J. G., Sutherland E. W. (1971) J. Biol. Chem. 246, 3841–3846 - PubMed
-
- Erneux C., Couchie D., Dumont J. E., Baraniak J., Stec W. J., Abbad E. G., Petridis G., Jastorff B. (1981) Eur. J. Biochem. 115, 503–510 - PubMed
-
- MacFarland R. T., Zelus B. D., Beavo J. A. (1991) J. Biol. Chem. 266, 136–142 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

