Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma
- PMID: 19632997
- PMCID: PMC2758018
- DOI: 10.1074/jbc.M109.019836
Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma
Abstract
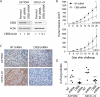
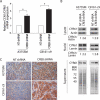
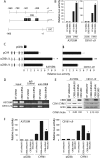
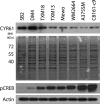
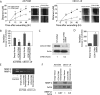
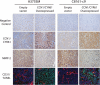
Metastatic progression of melanoma is associated with overexpression and activity of cAMP-response element-binding protein (CREB). However, the mechanism by which CREB contributes to tumor progression and metastasis remains unclear. Here, we demonstrate that stably silencing CREB expression in two human metastatic melanoma cell lines, A375SM and C8161-c9, suppresses tumor growth and experimental metastasis. Analysis of cDNA microarrays revealed that CREB silencing leads to increased expression of cysteine-rich protein 61 (CCN1/CYR61) known to mediate adhesion, chemostasis, survival, and angiogenesis. Promoter analysis and chromatin immunoprecipitation assays demonstrated that CREB acts as a negative regulator of CCN1/CYR61 transcription by directly binding to its promoter. Re-expression of CREB in CREB-silenced cells rescued the low CCN1/CYR61 expression phenotype. CCN1/CYR61 overexpression resulted in reduced tumor growth and metastasis and inhibited the activity of matrix metalloproteinase-2. Furthermore, its overexpression decreased melanoma cell motility and invasion through Matrigel, which was abrogated by silencing CCN1/CYR61 in low metastatic melanoma cells. Moreover, a significant decrease in angiogenesis as well as an increase in apoptosis was seen in tumors overexpressing CCN1/CYR61. Our results demonstrate that CREB promotes melanoma growth and metastasis by down-regulating CCN1/CYR61 expression, which acts as a suppressor of melanoma cell motility, invasion and angiogenesis.
Figures

References
-
- de Gruijl F. R. (1999) Eur. J. Cancer 35, 2003–2009 - PubMed
-
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M. J. (2008) CA Cancer J. Clin. 58, 71–96 - PubMed
-
- de Braud F., Khayat D., Kroon B. B., Valdagni R., Bruzzi P., Cascinelli N. (2003) Crit. Rev. Oncol. Hematol. 47, 35–63 - PubMed
-
- Smith A. P., Weeraratna A. T., Spears J. R., Meltzer P. S., Becker D. (2004) Cancer Biol. Ther. 3, 104–109 - PubMed
-
- McDonald S. L., Edington H. D., Kirkwood J. M., Becker D. (2004) Cancer Biol. Ther. 3, 110–120 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

