A paradoxical protective role for the proinflammatory peptide substance P receptor (NK1R) in acute hyperoxic lung injury
- PMID: 19633070
- PMCID: PMC2770780
- DOI: 10.1152/ajplung.90509.2008
A paradoxical protective role for the proinflammatory peptide substance P receptor (NK1R) in acute hyperoxic lung injury
Abstract
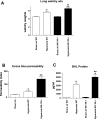
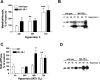
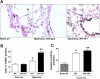
The neuropeptide substance P manifests its biological functions through ligation of a G protein-coupled receptor, the NK1R. Mice with targeted deletion of this receptor reveal a preponderance of proinflammatory properties resulting from ligand activation, demonstrating a neurogenic component to multiple forms of inflammation and injury. We hypothesized that NK1R deficiency would afford a similar protection from inflammation associated with hyperoxia. Counter to our expectations, however, NK1R-/- animals suffered significantly worse lung injury compared with wild-type mice following exposure to 90% oxygen. Median survival was shortened to 84 h for NK1R-/- mice from 120 h for wild-type animals. Infiltration of inflammatory cells into the lungs was significantly increased; NK1R-/- animals also exhibited increased pulmonary edema, hemorrhage, and bronchoalveolar lavage fluid protein levels. TdT-mediated dUTP nick end labeling (TUNEL) staining was significantly elevated in NK1R-/- animals following hyperoxia. Furthermore, induction of metallothionein and Na(+)-K(+)-ATPase was accelerated in NK1R-/- compared with wild-type mice, consistent with increased oxidative injury and edema. In cultured mouse lung epithelial cells in 95% O(2), however, addition of substance P promoted cell death, suggesting the neurogenic component of hyperoxic lung injury is mediated by additional mechanisms in vivo. Release of bioactive constituents including substance P from sensory neurons results from activation of the vanilloid receptor, TRPV1. In mice with targeted deletion of the TRPV1 gene, acute hyperoxic injury is attenuated relative to NK1R-/- animals. Our findings thus reveal a major neurogenic mechanism in acute hyperoxic lung injury and demonstrate concerted actions of sensory neurotransmitters revealing significant protection for NK1R-mediated functions.
Figures



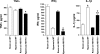


References
-
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004 - PubMed
-
- Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 19: 573–581, 1998 - PubMed
-
- Bill A, Stjernschantz J, Mandahl A, Brodin E, Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scand 106: 371–373, 1979 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

