Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth
- PMID: 19633169
- PMCID: PMC2730401
- DOI: 10.1242/dev.038679
Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth
Abstract
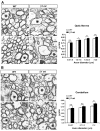
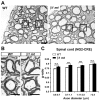
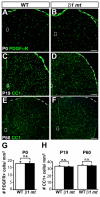
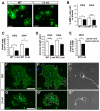
Oligodendrocytes in the central nervous system (CNS) produce myelin sheaths that insulate axons to ensure fast propagation of action potentials. beta1 integrins regulate the myelination of peripheral nerves, but their function during the myelination of axonal tracts in the CNS is unclear. Here we show that genetically modified mice lacking beta1 integrins in the CNS present a deficit in myelination but no defects in the development of the oligodendroglial lineage. Instead, in vitro data show that beta1 integrins regulate the outgrowth of myelin sheaths. Oligodendrocytes derived from mutant mice are unable to efficiently extend myelin sheets and fail to activate AKT (also known as AKT1), a kinase that is crucial for axonal ensheathment. The inhibition of PTEN, a negative regulator of AKT, or the expression of a constitutively active form of AKT restores myelin outgrowth in cultured beta1-deficient oligodendrocytes. Our data suggest that beta1 integrins play an instructive role in CNS myelination by promoting myelin wrapping in a process that depends on AKT.
Figures




Similar articles
-
Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS.J Neurosci. 2016 Apr 20;36(16):4506-21. doi: 10.1523/JNEUROSCI.3521-15.2016. J Neurosci. 2016. PMID: 27098694 Free PMC article.
-
Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination.J Neurosci. 2006 Jul 19;26(29):7665-73. doi: 10.1523/JNEUROSCI.0444-06.2006. J Neurosci. 2006. PMID: 16855094 Free PMC article.
-
Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation.Dev Biol. 2013 Jun 15;378(2):94-106. doi: 10.1016/j.ydbio.2013.03.023. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583582 Free PMC article.
-
Oligodendrocytes: Myelination and Axonal Support.Cold Spring Harb Perspect Biol. 2015 Jun 22;8(1):a020479. doi: 10.1101/cshperspect.a020479. Cold Spring Harb Perspect Biol. 2015. PMID: 26101081 Free PMC article. Review.
-
The cell biology of CNS myelination.Curr Opin Neurobiol. 2016 Aug;39:93-100. doi: 10.1016/j.conb.2016.04.013. Epub 2016 May 3. Curr Opin Neurobiol. 2016. PMID: 27152449 Free PMC article. Review.
Cited by
-
Laminin regulates oligodendrocyte development and myelination.Glia. 2022 Mar;70(3):414-429. doi: 10.1002/glia.24117. Epub 2021 Nov 12. Glia. 2022. PMID: 34773273 Free PMC article. Review.
-
Integrin signaling in oligodendrocytes and its importance in CNS myelination.J Signal Transduct. 2011;2011:354091. doi: 10.1155/2011/354091. Epub 2010 Dec 20. J Signal Transduct. 2011. PMID: 21637375 Free PMC article.
-
SHP-2 promotes the maturation of oligodendrocyte precursor cells through Akt and ERK1/2 signaling in vitro.PLoS One. 2011;6(6):e21058. doi: 10.1371/journal.pone.0021058. Epub 2011 Jun 20. PLoS One. 2011. PMID: 21701583 Free PMC article.
-
Identification of hub genes in the subacute spinal cord injury in rats.BMC Neurosci. 2022 Aug 27;23(1):51. doi: 10.1186/s12868-022-00737-5. BMC Neurosci. 2022. PMID: 36030234 Free PMC article.
-
Discoidin domain receptor regulates ensheathment, survival and caliber of peripheral axons.Development. 2022 Dec 1;149(23):dev200636. doi: 10.1242/dev.200636. Epub 2022 Dec 13. Development. 2022. PMID: 36355066 Free PMC article.
References
-
- Benninger, Y., Colognato, H., Thurnherr, T., Franklin, R. J., Leone, D. P., Atanasoski, S., Nave, K. A., ffrench-Constant, C., Suter, U. and Relvas, J. B. (2006). Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26, 7665-7673. - PMC - PubMed
-
- Berti, C., Nodari, A., Wrabetz, L. and Feltri, M. L. (2006). Role of integrins in peripheral nerves and hereditary neuropathies. Neuromolecular Med. 8, 191-204. - PubMed
-
- Buttery, P. C. and ffrench-Constant, C. (1999). Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell. Neurosci. 14, 199-212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

