Wash functions downstream of Rho and links linear and branched actin nucleation factors
- PMID: 19633175
- PMCID: PMC2730411
- DOI: 10.1242/dev.035246
Wash functions downstream of Rho and links linear and branched actin nucleation factors
Abstract
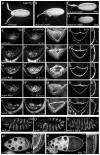
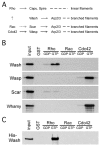
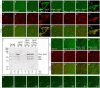
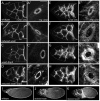
Wiskott-Aldrich Syndrome (WAS) family proteins are Arp2/3 activators that mediate the branched-actin network formation required for cytoskeletal remodeling, intracellular transport and cell locomotion. Wasp and Scar/WAVE, the two founding members of the family, are regulated by the GTPases Cdc42 and Rac, respectively. By contrast, linear actin nucleators, such as Spire and formins, are regulated by the GTPase Rho. We recently identified a third WAS family member, called Wash, with Arp2/3-mediated actin nucleation activity. We show that Drosophila Wash interacts genetically with Arp2/3, and also functions downstream of Rho1 with Spire and the formin Cappuccino to control actin and microtubule dynamics during Drosophila oogenesis. Wash bundles and crosslinks F-actin and microtubules, is regulated by Rho1, Spire and Arp2/3, and is essential for actin cytoskeleton organization in the egg chamber. Our results establish Wash and Rho as regulators of both linear- and branched-actin networks, and suggest an Arp2/3-mediated mechanism for how cells might coordinately regulate these structures.
Figures


Similar articles
-
Coordination of microtubule and microfilament dynamics by Drosophila Rho1, Spire and Cappuccino.Nat Cell Biol. 2006 Apr;8(4):367-76. doi: 10.1038/ncb1385. Epub 2006 Mar 5. Nat Cell Biol. 2006. PMID: 16518391 Free PMC article.
-
Regulatory interactions between two actin nucleators, Spire and Cappuccino.J Cell Biol. 2007 Oct 8;179(1):117-28. doi: 10.1083/jcb.200706196. J Cell Biol. 2007. PMID: 17923532 Free PMC article.
-
Abp1 utilizes the Arp2/3 complex activator Scar/WAVE in bristle development.J Cell Sci. 2012 Aug 1;125(Pt 15):3578-89. doi: 10.1242/jcs.101451. Epub 2012 Mar 30. J Cell Sci. 2012. PMID: 22467854
-
New insights into the regulation and cellular functions of the ARP2/3 complex.Nat Rev Mol Cell Biol. 2013 Jan;14(1):7-12. doi: 10.1038/nrm3492. Epub 2012 Dec 5. Nat Rev Mol Cell Biol. 2013. PMID: 23212475 Review.
-
Cellular functions of the Spir actin-nucleation factors.Trends Cell Biol. 2006 Sep;16(9):477-83. doi: 10.1016/j.tcb.2006.07.005. Epub 2006 Aug 9. Trends Cell Biol. 2006. PMID: 16901698 Review.
Cited by
-
Structures of actin-bound Wiskott-Aldrich syndrome protein homology 2 (WH2) domains of Spire and the implication for filament nucleation.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11757-62. doi: 10.1073/pnas.1005347107. Epub 2010 Jun 10. Proc Natl Acad Sci U S A. 2010. PMID: 20538977 Free PMC article.
-
Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair.Curr Biol. 2014 Jan 20;24(2):144-155. doi: 10.1016/j.cub.2013.11.048. Epub 2014 Jan 2. Curr Biol. 2014. PMID: 24388847 Free PMC article.
-
Autocrine insulin pathway signaling regulates actin dynamics in cell wound repair.PLoS Genet. 2020 Dec 11;16(12):e1009186. doi: 10.1371/journal.pgen.1009186. eCollection 2020 Dec. PLoS Genet. 2020. PMID: 33306674 Free PMC article.
-
Functional characterization of Wiskott-Aldrich syndrome protein and scar homolog (WASH), a bi-modular nucleation-promoting factor able to interact with biogenesis of lysosome-related organelle subunit 2 (BLOS2) and gamma-tubulin.J Biol Chem. 2010 May 28;285(22):16951-7. doi: 10.1074/jbc.M109.078501. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308062 Free PMC article.
-
WASH and the Arp2/3 complex regulate endosome shape and trafficking.Cytoskeleton (Hoboken). 2010 Mar;67(3):193-206. doi: 10.1002/cm.20437. Cytoskeleton (Hoboken). 2010. PMID: 20175130 Free PMC article.
References
-
- Berger, S., Schafer, G., Kesper, D. A., Holz, A., Eriksson, T., Palmer, R. H., Beck, L., Klambt, C., Renkawitz-Pohl, R. and Onel, S. F. (2008). WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J. Cell Sci. 121, 1303-1313. - PubMed
-
- Bione, S., Sala, C., Manzini, C., Arrigo, G., Zuffardi, O., Banfi, S., Borsani, G., Jonveaux, P., Philippe, C., Zuccotti, M. et al. (1998). A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am. J. Hum. Genet. 62, 533-541. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

