Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth
- PMID: 19633191
- PMCID: PMC2726404
- DOI: 10.1073/pnas.0901170106
Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth
Abstract
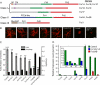
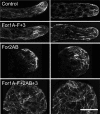
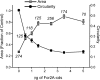
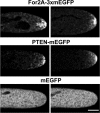
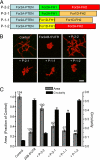
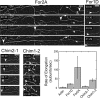
Formins are present in all eukaryotes and are essential for the creation of actin-based structures responsible for diverse cellular processes. Because multicellular organisms contain large formin gene families, establishing the physiological functions of formin isoforms has been difficult. Using RNAi, we analyzed the function of all 9 formin genes within the moss Physcomitrella patens. We show that plants lacking class II formins (For2) are severely stunted and composed of spherical cells with disrupted actin organization. In contrast, silencing of all other formins results in normal elongated cell morphology and actin organization. Consistent with a role in polarized growth, For2 are apically localized in growing cells. We show that an N-terminal phosphatase tensin (PTEN)-like domain mediates apical localization. The PTEN-like domain is followed by a conserved formin homology (FH)1-FH2 domain, known to promote actin polymerization. To determine whether apical localization of any FH1-FH2 domain mediates polarized growth, we performed domain swapping. We found that only the class II FH1-FH2, in combination with the PTEN-like domain, rescues polarized growth, because it cannot be replaced with a similar domain from a For1. We used in vitro polymerization assays to dissect the functional differences between these FH1-FH2 domains. We found that both the FH1 and the FH2 domains from For2 are required to mediate exceptionally rapid rates of actin filament elongation, much faster than any other known formin. Thus, our data demonstrate that rapid rates of actin elongation are critical for driving the formation of apical filamentous actin necessary for polarized growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Evangelista M, et al. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. - PubMed
-
- Feierbach B, Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol. 2001;11:1656–1665. - PubMed
-
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. - PubMed
-
- Higgs HN. Formin proteins: A domain-based approach. Trends Biochem Sci. 2005;30:342–353. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

