Myocardial interstitial fluid inhibits proliferation and cardiomyocyte differentiation in pluripotent embryonic stem cells
- PMID: 19633209
- PMCID: PMC2770771
- DOI: 10.1152/ajpheart.00172.2009
Myocardial interstitial fluid inhibits proliferation and cardiomyocyte differentiation in pluripotent embryonic stem cells
Abstract
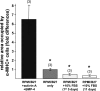
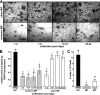
Several recent studies have demonstrated that the transplantation of pluripotent murine embryonic stem cells (mESCs) can improve or restore the function of infarcted myocardium. Although the extent of remuscularization and its contribution to the restoration of function are unclear, these outcomes are likely strongly influenced by factors in the infarcted and/or ischemic environment. As an initial step toward understanding how the ischemic environment of host myocardium affects transplanted pluripotent cells, we have taken a reductionist approach wherein mESCs are cultured in medium containing ischemic myocardial interstitial fluid (iMIF). iMIF is generated in canine myocardium during eight hourly episodes of transient ischemia and collected on a daily basis, over a 24-day collection period. iMIF strongly reduced the numbers of pluripotent mESCs after 11 days in culture. This inhibitory effect, which was most pronounced for iMIF pools from early time points of the 24-day collection period, resulted from an inhibition of cell proliferation. iMIF also inhibited the differentiation of pluripotent mESCs into cardiomyocytes. By contrast, the expression of vascular smooth muscle and endothelial cell markers was relatively unaffected, consistent with previous findings that iMIF promotes angiogenesis. Taken together, these results suggest that whereas the ischemic/infarcted environment is favorable to stem cell-mediated angiogenesis, it is hostile to cardiac myogenesis. These findings also imply that observations of mESC-mediated improvement of cardiac function after transplantation of pluripotent cells do not reflect remuscularization.
Figures



Similar articles
-
Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells.Anat Rec A Discov Mol Cell Evol Biol. 2006 Nov;288(11):1216-24. doi: 10.1002/ar.a.20388. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 17004246 Free PMC article.
-
Rapamycin and CHIR99021 Coordinate Robust Cardiomyocyte Differentiation From Human Pluripotent Stem Cells Via Reducing p53-Dependent Apoptosis.J Am Heart Assoc. 2017 Oct 2;6(10):e005295. doi: 10.1161/JAHA.116.005295. J Am Heart Assoc. 2017. PMID: 28971953 Free PMC article.
-
Characterization of rat very small embryonic-like stem cells and cardiac repair after cell transplantation for myocardial infarction.Stem Cells Dev. 2012 May 20;21(8):1367-79. doi: 10.1089/scd.2011.0280. Epub 2011 Oct 27. Stem Cells Dev. 2012. PMID: 22032240
-
Progress and Challenges of Amniotic Fluid Derived Stem Cells in Therapy of Ischemic Heart Disease.Int J Mol Sci. 2020 Dec 24;22(1):102. doi: 10.3390/ijms22010102. Int J Mol Sci. 2020. PMID: 33374215 Free PMC article. Review.
-
Developmental origins of hypertrophic cardiomyopathy phenotypes: a unifying hypothesis.Nat Rev Cardiol. 2009 Apr;6(4):317-21. doi: 10.1038/nrcardio.2009.9. Nat Rev Cardiol. 2009. PMID: 19352336 Review.
Cited by
-
ERG deregulation induces PIM1 over-expression and aneuploidy in prostate epithelial cells.PLoS One. 2011;6(11):e28162. doi: 10.1371/journal.pone.0028162. Epub 2011 Nov 30. PLoS One. 2011. PMID: 22140532 Free PMC article.
References
-
- American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 - PubMed
-
- Guide for the Care and Use of Laboratory Animals Washington, DC: National Academy Press, 1996
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068–14073, 2007 - PMC - PubMed
-
- Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 16: 1558–1566, 2002 - PubMed
-
- Behfar A, Hodgson DM, Zingman LV, Perez-Terzic C, Yamada S, Kane GC, Alekseev AE, Puceat M, Terzic A. Administration of allogenic stem cells dosed to secure cardiogenesis and sustained infarct repair. Ann NY Acad Sci 1049: 189–198, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

