Auxin stimulates its own transport by shaping actin filaments
- PMID: 19633235
- PMCID: PMC2736007
- DOI: 10.1104/pp.109.140111
Auxin stimulates its own transport by shaping actin filaments
Abstract
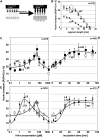
The directional transport of the plant hormone auxin has been identified as central element of axis formation and patterning in plants. This directionality of transport depends on gradients, across the cell, of auxin-efflux carriers that continuously cycle between plasma membrane and intracellular compartments. This cycling has been proposed to depend on actin filaments. However, the role of actin for the polarity of auxin transport has been disputed. The organization of actin, in turn, has been shown to be under control of auxin. By overexpression of the actin-binding protein talin, we have generated transgenic rice (Oryza sativa) lines, where actin filaments are bundled to variable extent and, in consequence, display a reduced dynamics. We show that this bundling of actin filaments correlates with impaired gravitropism and reduced longitudinal transport of auxin. We can restore a normal actin configuration by addition of exogenous auxins and restore gravitropism as well as polar auxin transport. This rescue is mediated by indole-3-acetic acid and 1-naphthyl acetic acid but not by 2,4-dichlorophenoxyacetic acid. We interpret these findings in the context of a self-referring regulatory circuit between polar auxin transport and actin organization. This circuit might contribute to the self-amplification of auxin transport that is a central element in current models of auxin-dependent patterning.
Figures




Similar articles
-
Probing the actin-auxin oscillator.Plant Signal Behav. 2010 Feb;5(2):94-8. doi: 10.4161/psb.5.2.10337. Epub 2010 Feb 15. Plant Signal Behav. 2010. PMID: 20023411 Free PMC article. Review.
-
Actin is involved in auxin-dependent patterning.Plant Physiol. 2007 Apr;143(4):1695-704. doi: 10.1104/pp.106.094052. Epub 2007 Mar 2. Plant Physiol. 2007. PMID: 17337532 Free PMC article.
-
VLN2 Regulates Plant Architecture by Affecting Microfilament Dynamics and Polar Auxin Transport in Rice.Plant Cell. 2015 Oct;27(10):2829-45. doi: 10.1105/tpc.15.00581. Epub 2015 Oct 20. Plant Cell. 2015. PMID: 26486445 Free PMC article.
-
The actin cytoskeleton may control the polar distribution of an auxin transport protein.Gravit Space Biol Bull. 2000 Jun;13(2):75-83. Gravit Space Biol Bull. 2000. PMID: 11543284
-
Keeping it all together: auxin-actin crosstalk in plant development.J Exp Bot. 2015 Aug;66(16):4983-98. doi: 10.1093/jxb/erv308. Epub 2015 Jun 17. J Exp Bot. 2015. PMID: 26085676 Review.
Cited by
-
Auxin protects Arabidopsis thaliana cell suspension cultures from programmed cell death induced by the cellulose biosynthesis inhibitors thaxtomin A and isoxaben.BMC Plant Biol. 2019 Nov 21;19(1):512. doi: 10.1186/s12870-019-2130-2. BMC Plant Biol. 2019. PMID: 31752698 Free PMC article.
-
Effects of a coumarin derivative, 4-methylumbelliferone, on seed germination and seedling establishment in Arabidopsis.J Chem Ecol. 2011 Aug;37(8):880-90. doi: 10.1007/s10886-011-9987-3. Epub 2011 Jun 29. J Chem Ecol. 2011. PMID: 21713565
-
Auxin-induced actin cytoskeleton rearrangements require AUX1.New Phytol. 2020 Apr;226(2):441-459. doi: 10.1111/nph.16382. Epub 2020 Feb 11. New Phytol. 2020. PMID: 31859367 Free PMC article.
-
Root apex transition zone as oscillatory zone.Front Plant Sci. 2013 Oct 2;4:354. doi: 10.3389/fpls.2013.00354. eCollection 2013. Front Plant Sci. 2013. PMID: 24106493 Free PMC article.
-
Actin as deathly switch? How auxin can suppress cell-death related defence.PLoS One. 2015 May 1;10(5):e0125498. doi: 10.1371/journal.pone.0125498. eCollection 2015. PLoS One. 2015. PMID: 25933033 Free PMC article.
References
-
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4 57–62 - PubMed
-
- Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8 494–500 - PubMed
-
- Butler JH, Hu S, Brady SR, Dixon MW, Muday GK (1998) In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J 13 291–301 - PubMed
-
- Cande WZ, Goldsmith MHM, Ray PM (1973) Polar auxin transport and auxin-induced elongation in the absence of cytoplasmic streaming. Planta 111 279–296 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

