Slt2 and Rim101 contribute independently to the correct assembly of the chitin ring at the budding yeast neck in Saccharomyces cerevisiae
- PMID: 19633265
- PMCID: PMC2747826
- DOI: 10.1128/EC.00153-09
Slt2 and Rim101 contribute independently to the correct assembly of the chitin ring at the budding yeast neck in Saccharomyces cerevisiae
Abstract
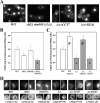
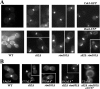
In Saccharomyces cerevisiae, the simultaneous absence of Slt2 and Rim101 prevents growth in nonosmotically stabilized media (F. Castrejon et al., Eukaryot. Cell 5:507-517, 2006). The double mutant slt2Delta rim101Delta displays altered chitin rings, together with a significant reduction in the overall levels of chitin. Cultures of this mutant lyse upon transfer to nonosmotically stabilized media, mostly through the bud, and such lysis is partially prevented by deletion of the chitinase gene (CTS1). Growth of the slt2Delta rim101Delta double mutant was restored by the overexpression of the GFA1 or CCT7 genes, which code for two biologically unrelated proteins. Further characterization of the mutant and its suppressors indicated that both Slt2 and Rim101 were independently required for the correct assembly of the septum machinery and that their concomitant absence reduced Chs3 accumulation at the neck, leading to lower levels of chitin. GFA1 overexpression, as well as the addition of glucosamine to the growth medium, specifically suppressed the growth defects by activating chitin synthesis at the neck and restoring the normal assembly of the chitin ring. In contrast, overexpression of CCT7, a Cct chaperonin subunit, alleviated the defect in the septum machinery without affecting chitin synthesis. Both suppressors thus act by reducing neck fragility through different mechanisms and allow growth in nonstabilized media. This work reports new roles for Slt2 and Rim101 in septum formation in budding yeast and confirms the homeostatic role of the chitin ring in the maintenance of neck integrity during cell division.
Figures




Similar articles
-
The RIM101 pathway contributes to yeast cell wall assembly and its function becomes essential in the absence of mitogen-activated protein kinase Slt2p.Eukaryot Cell. 2006 Mar;5(3):507-17. doi: 10.1128/EC.5.3.507-517.2006. Eukaryot Cell. 2006. PMID: 16524906 Free PMC article.
-
Saccharomyces cerevisiae Bni4p directs the formation of the chitin ring and also participates in the correct assembly of the septum structure.Microbiology (Reading). 2004 Oct;150(Pt 10):3229-41. doi: 10.1099/mic.0.27352-0. Microbiology (Reading). 2004. PMID: 15470103
-
Role for lipid signaling and the cell integrity MAP kinase cascade in yeast septum biogenesis.Curr Genet. 2003 May;43(2):71-8. doi: 10.1007/s00294-003-0380-9. Epub 2003 Mar 7. Curr Genet. 2003. PMID: 12695846
-
A walk-through MAPK structure and functionality with the 30-year-old yeast MAPK Slt2.Int Microbiol. 2021 Nov;24(4):531-543. doi: 10.1007/s10123-021-00183-z. Epub 2021 May 15. Int Microbiol. 2021. PMID: 33993419 Review.
-
Biosynthesis of cell wall and septum during yeast growth.Arch Med Res. 1993 Autumn;24(3):301-3. Arch Med Res. 1993. PMID: 8298281 Review.
Cited by
-
Maintaining protein homeostasis: early and late endosomal dual recycling for the maintenance of intracellular pools of the plasma membrane protein Chs3.Mol Biol Cell. 2016 Dec 15;27(25):4021-4032. doi: 10.1091/mbc.E16-04-0239. Epub 2016 Oct 19. Mol Biol Cell. 2016. PMID: 27798229 Free PMC article.
-
An expanded cell wall damage signaling network is comprised of the transcription factors Rlm1 and Sko1 in Candida albicans.PLoS Genet. 2020 Jul 8;16(7):e1008908. doi: 10.1371/journal.pgen.1008908. eCollection 2020 Jul. PLoS Genet. 2020. PMID: 32639995 Free PMC article.
-
The kinase Isr1 negatively regulates hexosamine biosynthesis in S. cerevisiae.PLoS Genet. 2020 Jun 24;16(6):e1008840. doi: 10.1371/journal.pgen.1008840. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32579556 Free PMC article.
-
Slt2 Is Required to Activate ER-Stress-Protective Mechanisms through TORC1 Inhibition and Hexosamine Pathway Activation.J Fungi (Basel). 2022 Jan 18;8(2):92. doi: 10.3390/jof8020092. J Fungi (Basel). 2022. PMID: 35205847 Free PMC article.
-
The Role of the Cell Integrity Pathway in Septum Assembly in Yeast.J Fungi (Basel). 2021 Sep 6;7(9):729. doi: 10.3390/jof7090729. J Fungi (Basel). 2021. PMID: 34575767 Free PMC article. Review.
References
-
- Andrews, P. D., and M. J. Stark. 2000. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci. 113:507-520. - PubMed
-
- Bermejo, C., E. Rodríguez, R. García, J. M. Rodríguez-Peña, M. L. Rodríguez de la Concepción, C. Rivas, P. Arias, C. Nombela, F. Posas, and J. Arroyo. 2008. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 19:1113-1124. - PMC - PubMed
-
- Bulawa, C. E., M. Slater, E. Cabib, J. Au-Young, A. Sburlati, W. L. J. Adair, and P. W. Robbins. 1986. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell 46:213-225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

