How a cell crawls and the role of cortical myosin II
- PMID: 19633268
- PMCID: PMC2747829
- DOI: 10.1128/EC.00121-09
How a cell crawls and the role of cortical myosin II
Abstract
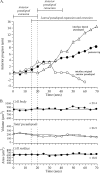
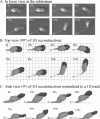
The movements of Dictyostelium discoideum amoebae translocating on a glass surface in the absence of chemoattractant have been reconstructed at 5-second intervals and motion analyzed by employing 3D-DIAS software. A morphometric analysis of pseudopods, the main cell body, and the uropod provides a comprehensive description of the basic motile behavior of a cell in four dimensions (4D), resulting in a list of 18 characteristics. A similar analysis of the myosin II phosphorylation mutant 3XASP reveals a role for the cortical localization of myosin II in the suppression of lateral pseudopods, formation of the uropod, cytoplasmic distribution of cytoplasm in the main cell body, and efficient motility. The results of the morphometric analysis suggest that pseudopods, the main cell body, and the uropod represent three motility compartments that are coordinated for efficient translocation. It provides a contextual framework for interpreting the effects of mutations, inhibitors, and chemoattractants on the basic motile behavior of D. discoideum. The generality of the characteristics of the basic motile behavior of D. discoideum must now be tested by similar 4D analyses of the motility of amoeboid cells of higher eukaryotic cells, in particular human polymorphonuclear leukocytes.
Figures









References
-
- Andrew, N., and R. H. Insall. 2007. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9:193-200. - PubMed
-
- Bonner, J. T., D. S. Barkley, E. M. Hall, T. M. Konijn, J. W. Mason, G. O'Keefe III, and P. B. Wolfe. 1969. Acrasin, acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev. Biol. 20:72-87. - PubMed
-
- Bosgraaf, L., and P. J. van Haastert. 2006. The regulation of myosin II in Dictyostelium. Eur. J. Cell Biol. 85:969-979. - PubMed
-
- Bottino, D., A. Mogilner, T. Roberts, M. Stewart, and G. Oster. 2002. How nematode sperm crawl. J. Cell Sci. 115:367-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

