Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate
- PMID: 19633296
- PMCID: PMC2785382
- DOI: 10.1074/jbc.M109.013706
Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate
Abstract
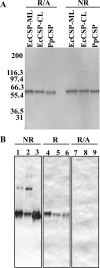
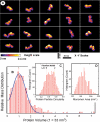
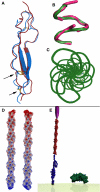
The Plasmodium falciparum circumsporozoite protein (CSP) is critical for sporozoite function and invasion of hepatocytes. Given its critical nature, a phase III human CSP malaria vaccine trial is ongoing. The CSP is composed of three regions as follows: an N terminus that binds heparin sulfate proteoglycans, a four amino acid repeat region (NANP), and a C terminus that contains a thrombospondin-like type I repeat (TSR) domain. Despite the importance of CSP, little is known about its structure. Therefore, recombinant forms of CSP were produced by expression in both Escherichia coli (Ec) and then refolded (EcCSP) or in the methylotrophic yeast Pichia pastoris (PpCSP) for structural analyses. To analyze the TSR domain of recombinant CSP, conformation-dependent monoclonal antibodies that recognized unfixed P. falciparum sporozoites and inhibited sporozoite invasion of HepG2 cells in vitro were identified. These monoclonal antibodies recognized all recombinant CSPs, indicating the recombinant CSPs contain a properly folded TSR domain structure. Characterization of both EcCSP and PpCSP by dynamic light scattering and velocity sedimentation demonstrated that both forms of CSP appeared as highly extended proteins (R(h) 4.2 and 4.58 nm, respectively). Furthermore, high resolution atomic force microscopy revealed flexible, rod-like structures with a ribbon-like appearance. Using this information, we modeled the NANP repeat and TSR domain of CSP. Consistent with the biochemical and biophysical results, the repeat region formed a rod-like structure about 21-25 nm in length and 1.5 nm in width. Thus native CSP appears as a glycosylphosphatidylinositol-anchored, flexible rod-like protein on the sporozoite surface.
Figures




References
-
- Clyde D. F., Most H., McCarthy V. C., Vanderberg J. P. (1973) Am. J. Med. Sci. 266, 169–177 - PubMed
-
- Nussenzweig R. S., Vanderberg J., Most H., Orton C. (1967) Nature 216, 160–162 - PubMed
-
- Nussenzweig R. S., Vanderberg J. P., Most H., Orton C. (1969) Nature 222, 488–489 - PubMed
-
- Hollingdale M. R., Zavala F., Nussenzweig R. S., Nussenzweig V. (1982) J. Immunol. 128, 1929–1930 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

