Alternative splicing of Na(V)1.7 exon 5 increases the impact of the painful PEPD mutant channel I1461T
- PMID: 19633428
- PMCID: PMC2856339
- DOI: 10.4161/chan.3.4.9341
Alternative splicing of Na(V)1.7 exon 5 increases the impact of the painful PEPD mutant channel I1461T
Abstract
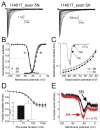
Alternative splicing is known to alter pharmacological sensitivities, kinetics, channel distribution under pathological conditions, and developmental regulation of VGSCs. Mutations that alter channel properties in Na(V)1.7 have been genetically implicated in patients with bouts of extreme pain classified as inherited erythromelalgia (IEM) or paroxysmal extreme pain disorder (PEPD). Furthermore, patients with IEM or PEPD report differential age onsets. A recent study reported that alternative splicing of Na(V)1.7 exon 5 affects ramp current properties. Since IEM and PEPD mutations also alter Na(V)1.7 ramp current properties we speculated that alternative splicing might impact the functional consequences of IEM or PEPD mutations. We compared the effects alternative splicing has on the biophysical properties of Na(V)1.7 wild-type, IEM (I136V) and PEPD (I1461T) channels. Our major findings demonstrate that although the 5A splice variant of the IEM channel had no functional impact, the 5A splice variant of the PEPD channel significantly hyperpolarized the activation curve, slowed deactivation and closed-state inactivation, shifted the ramp current activation to more hyperpolarized potentials, and increased ramp current amplitude. We hypothesize a D1/S3-S4 charged residue difference between the 5N (Asn) and the 5A (Asp) variants within the coding region of exon 5 may contribute to shifts in channel activation and deactivation. Taken together, the additive effects observed on ramp currents from exon 5 splicing and the PEPD mutation (I1461T) are likely to impact the disease phenotype and may offer insight into how alternative splicing may affect specific intramolecular interactions critical for voltage-dependent gating.
Figures





Similar articles
-
NaV1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders.J Neurosci. 2008 Oct 22;28(43):11079-88. doi: 10.1523/JNEUROSCI.3443-08.2008. J Neurosci. 2008. PMID: 18945915 Free PMC article.
-
Mutations at opposite ends of the DIII/S4-S5 linker of sodium channel Na V 1.7 produce distinct pain disorders.Mol Pain. 2010 Apr 29;6:24. doi: 10.1186/1744-8069-6-24. Mol Pain. 2010. PMID: 20429905 Free PMC article.
-
Paroxysmal extreme pain disorder mutations within the D3/S4-S5 linker of Nav1.7 cause moderate destabilization of fast inactivation.J Physiol. 2008 Sep 1;586(17):4137-53. doi: 10.1113/jphysiol.2008.154906. Epub 2008 Jul 3. J Physiol. 2008. PMID: 18599537 Free PMC article.
-
Genetics and molecular pathophysiology of Na(v)1.7-related pain syndromes.Adv Genet. 2008;63:85-110. doi: 10.1016/S0065-2660(08)01004-3. Adv Genet. 2008. PMID: 19185186 Review.
-
Pain disorders and erythromelalgia caused by voltage-gated sodium channel mutations.Curr Neurol Neurosci Rep. 2012 Feb;12(1):76-83. doi: 10.1007/s11910-011-0233-8. Curr Neurol Neurosci Rep. 2012. PMID: 21984269 Review.
Cited by
-
Sodium channelopathies and pain.Pflugers Arch. 2010 Jul;460(2):249-63. doi: 10.1007/s00424-009-0779-3. Epub 2010 Jan 26. Pflugers Arch. 2010. PMID: 20101409 Review.
-
Alternative splicing modulates inactivation of type 1 voltage-gated sodium channels by toggling an amino acid in the first S3-S4 linker.J Biol Chem. 2011 Oct 21;286(42):36700-8. doi: 10.1074/jbc.M111.250225. Epub 2011 Sep 2. J Biol Chem. 2011. PMID: 21890636 Free PMC article.
-
Pain as a channelopathy.J Clin Invest. 2010 Nov;120(11):3745-52. doi: 10.1172/JCI43158. Epub 2010 Nov 1. J Clin Invest. 2010. PMID: 21041956 Free PMC article. Review.
-
Genes, molecules and patients--emerging topics to guide clinical pain research.Eur J Pharmacol. 2013 Sep 15;716(1-3):188-202. doi: 10.1016/j.ejphar.2013.01.069. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23500200 Free PMC article. Review.
-
Conservation of alternative splicing in sodium channels reveals evolutionary focus on release from inactivation and structural insights into gating.J Physiol. 2017 Aug 15;595(16):5671-5685. doi: 10.1113/JP274693. Epub 2017 Jul 18. J Physiol. 2017. PMID: 28621020 Free PMC article.
References
-
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. 1952. BullMathBiol. 1990;52:25–71. - PubMed
-
- Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–61. - PubMed
-
- Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. - PubMed
-
- Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–10. - PubMed
-
- Doyle DA, Morais CJ, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, Mackinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
