Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study
- PMID: 19633577
- PMCID: PMC3258511
- DOI: 10.1097/MPG.0b013e31819bcac4
Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study
Abstract
Objectives: : A multicenter, double-blind study was conducted to evaluate the safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate ulcerative colitis (UC).
Patients and methods: : Sixty-eight patients, 5 to 17 years of age, with mild-to-moderate active UC based on the modified Sutherland UC activity index (MUCAI) were randomized to receive oral balsalazide 2.25 or 6.75 g/day for 8 weeks. The primary endpoint was clinical improvement (reduction of the MUCAI score by > or =3 points from baseline). Clinical remission (MUCAI score of 0 or 1 for stool frequency) and histological improvement after 8 weeks were also assessed. Pharmacokinetic parameters for balsalazide, 5-aminosalicylic acid, and N-acetyl-5-aminosalicylic acid were determined at 2 weeks. Adverse events and laboratory changes were monitored throughout the study.
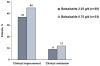
Results: : Clinical improvement was achieved by 45% and 37% of patients and clinical remission by 12% and 9% of patients receiving 6.75 and 2.25 g/day, respectively. Improvement in histologic grade was achieved by 8 of 16 (50%) and 3 of 10 (30%) patients receiving 6.75 and 2.25 g/day, respectively. No significant differences were seen in efficacy. Pharmacokinetics in 12 patients were characterized by large interpatient variability and low systemic exposure. Adverse events were similar between the treatment groups, the most common being headache and abdominal pain. No clinically significant changes were observed in laboratory values, including those indicative of hepatic or renal toxicity.
Conclusions: : Balsalazide is well tolerated and improves the signs and symptoms of mild-to-moderate active UC in pediatric patients 5 to 17 years of age.
Figures
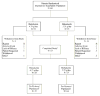
Patient withdrawn by investigator because rectal biopsy slides from colonoscopy showed mild dysplasia
One patient did not meet inclusion criteria, another patient was noncompliant
atients who completed the study had colonoscopies performed at screening and week 8
Patients were excluded from per-protocol population for one or more of the following reasons: Violations of inclusion/exclusion criteria (n = 12); <70% compliant (n = 7); > 30 days between colonoscopy and start of study medication (n = 3); used prohibited medications (n = 2); did not provide complete week 8 MUCAl data (n = 4); week 8 MUCAI data not collected between days 42 and 70 (n = 5)
Patients were excluded from per-protocol population for one or more of the following reasons. Violations of inclusion/exclusion criteria (n = 8); <70% compliant (n = 12); did not meet baseline MUCAI requirements (n = 2); >30 days between colonoscopy and start of study medication (n = 5); used prohibited medications (n = 2); did not provide complete week 8 MUCAI data (n = 8); week 8 MUCAI data not collected between days 42 and 70 (n = 10)
Similar articles
-
Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis.Am J Gastroenterol. 2002 Dec;97(12):3078-86. doi: 10.1111/j.1572-0241.2002.07103.x. Am J Gastroenterol. 2002. PMID: 12492193 Clinical Trial.
-
A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis.Am J Gastroenterol. 2002 Jun;97(6):1398-407. doi: 10.1111/j.1572-0241.2002.05781.x. Am J Gastroenterol. 2002. PMID: 12094857 Clinical Trial.
-
Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group.Gastroenterology. 1998 Jan;114(1):15-22. doi: 10.1016/s0016-5085(98)70627-4. Gastroenterology. 1998. PMID: 9428213 Clinical Trial.
-
Balsalazide: a review of its therapeutic use in mild-to-moderate ulcerative colitis.Drugs. 2002;62(11):1689-705. doi: 10.2165/00003495-200262110-00010. Drugs. 2002. PMID: 12109930 Review.
-
Comparison of mesalazine and balsalazide in induction and maintenance of remission in patients with ulcerative colitis: a meta-analysis.Dig Dis Sci. 2009 Apr;54(4):712-21. doi: 10.1007/s10620-008-0428-2. Epub 2008 Aug 6. Dig Dis Sci. 2009. PMID: 18683049 Review.
Cited by
-
Colitis and Crohn's Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease.Intest Res. 2020 Oct;18(4):355-378. doi: 10.5217/ir.2019.09176. Epub 2020 Jul 13. Intest Res. 2020. PMID: 32646198 Free PMC article. Review.
-
Pediatric ulcerative colitis: current treatment approaches including role of infliximab.Biologics. 2012;6:125-34. doi: 10.2147/BTT.S31833. Epub 2012 Jun 5. Biologics. 2012. PMID: 22740771 Free PMC article.
-
Inflammatory Bowel Disease in Children and Adolescents.JAMA Pediatr. 2015 Nov;169(11):1053-60. doi: 10.1001/jamapediatrics.2015.1982. JAMA Pediatr. 2015. PMID: 26414706 Free PMC article. Review.
-
Randomized clinical trial: pharmacokinetics and safety of multimatrix mesalamine for treatment of pediatric ulcerative colitis.Drug Des Devel Ther. 2016 Feb 4;10:593-607. doi: 10.2147/DDDT.S95316. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 26893546 Free PMC article. Clinical Trial.
-
Inflammatory Bowel Disease in Childhood and Adolescence.Dtsch Arztebl Int. 2017 May 12;114(19):331-338. doi: 10.3238/arztebl.2017.0331. Dtsch Arztebl Int. 2017. PMID: 28597827 Free PMC article. Review.
References
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. - PubMed
-
- Loftus CG, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, III, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. - PubMed
-
- Bousvaros A, Sylvester F, Kugathasan S, Szigethy E, Fiocchi C, Colletti R, Otley A, Amre D, Ferry G, Czinn SJ, Splawski JB, Oliva-Hemker M, Hyams JS, Faubion WA, Kirschner BS, Dubinsky MC. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. - PubMed
-
- Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006:CD000543. - PubMed
-
- Lim WC, Hanauer SB. Controversies with aminosalicylates in inflammatory bowel disease. Rev Gastroenterol Disord. 2004;4:104–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

