Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study
- PMID: 19633577
- PMCID: PMC3258511
- DOI: 10.1097/MPG.0b013e31819bcac4
Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study
Abstract
Objectives: : A multicenter, double-blind study was conducted to evaluate the safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate ulcerative colitis (UC).
Patients and methods: : Sixty-eight patients, 5 to 17 years of age, with mild-to-moderate active UC based on the modified Sutherland UC activity index (MUCAI) were randomized to receive oral balsalazide 2.25 or 6.75 g/day for 8 weeks. The primary endpoint was clinical improvement (reduction of the MUCAI score by > or =3 points from baseline). Clinical remission (MUCAI score of 0 or 1 for stool frequency) and histological improvement after 8 weeks were also assessed. Pharmacokinetic parameters for balsalazide, 5-aminosalicylic acid, and N-acetyl-5-aminosalicylic acid were determined at 2 weeks. Adverse events and laboratory changes were monitored throughout the study.
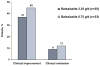
Results: : Clinical improvement was achieved by 45% and 37% of patients and clinical remission by 12% and 9% of patients receiving 6.75 and 2.25 g/day, respectively. Improvement in histologic grade was achieved by 8 of 16 (50%) and 3 of 10 (30%) patients receiving 6.75 and 2.25 g/day, respectively. No significant differences were seen in efficacy. Pharmacokinetics in 12 patients were characterized by large interpatient variability and low systemic exposure. Adverse events were similar between the treatment groups, the most common being headache and abdominal pain. No clinically significant changes were observed in laboratory values, including those indicative of hepatic or renal toxicity.
Conclusions: : Balsalazide is well tolerated and improves the signs and symptoms of mild-to-moderate active UC in pediatric patients 5 to 17 years of age.
Figures
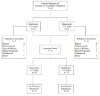
Patient withdrawn by investigator because rectal biopsy slides from colonoscopy showed mild dysplasia
One patient did not meet inclusion criteria, another patient was noncompliant
atients who completed the study had colonoscopies performed at screening and week 8
Patients were excluded from per-protocol population for one or more of the following reasons: Violations of inclusion/exclusion criteria (n = 12); <70% compliant (n = 7); > 30 days between colonoscopy and start of study medication (n = 3); used prohibited medications (n = 2); did not provide complete week 8 MUCAl data (n = 4); week 8 MUCAI data not collected between days 42 and 70 (n = 5)
Patients were excluded from per-protocol population for one or more of the following reasons. Violations of inclusion/exclusion criteria (n = 8); <70% compliant (n = 12); did not meet baseline MUCAI requirements (n = 2); >30 days between colonoscopy and start of study medication (n = 5); used prohibited medications (n = 2); did not provide complete week 8 MUCAI data (n = 8); week 8 MUCAI data not collected between days 42 and 70 (n = 10)
References
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. - PubMed
-
- Loftus CG, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, III, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. - PubMed
-
- Bousvaros A, Sylvester F, Kugathasan S, Szigethy E, Fiocchi C, Colletti R, Otley A, Amre D, Ferry G, Czinn SJ, Splawski JB, Oliva-Hemker M, Hyams JS, Faubion WA, Kirschner BS, Dubinsky MC. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. - PubMed
-
- Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006:CD000543. - PubMed
-
- Lim WC, Hanauer SB. Controversies with aminosalicylates in inflammatory bowel disease. Rev Gastroenterol Disord. 2004;4:104–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

