The twin spot generator for differential Drosophila lineage analysis
- PMID: 19633664
- PMCID: PMC2720837
- DOI: 10.1038/nmeth.1349
The twin spot generator for differential Drosophila lineage analysis
Abstract
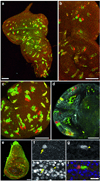
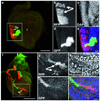
In Drosophila melanogaster, widely used mitotic recombination-based strategies generate mosaic flies with positive readout for only one daughter cell after division. To differentially label both daughter cells, we developed the twin spot generator (TSG) technique, which through mitotic recombination generates green and red twin spots that are detectable after the first cell division as single cells. We propose wide applications of TSG to lineage and genetic mosaic studies.
Figures

References
-
- Lee T, Luo L. Neuron. 1999;22:451–461. - PubMed
-
- Lee T, Luo L. Trends Neurosci. 2001;24:251–254. - PubMed
-
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Cell. 2004;117:107–116. - PubMed
-
- Spradling AC, Rubin GM. Science. 1982;218:341–347. - PubMed
-
- Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Cell. 2005;121:479–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

