A bushy cell network in the rat ventral cochlear nucleus
- PMID: 19634178
- PMCID: PMC2841288
- DOI: 10.1002/cne.22139
A bushy cell network in the rat ventral cochlear nucleus
Abstract
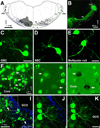
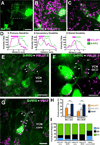
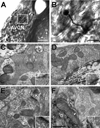
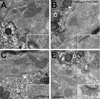
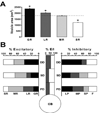
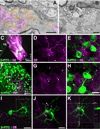
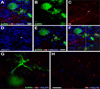
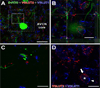
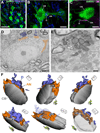
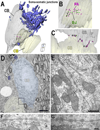
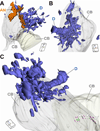
Geometry of the dendritic tree and synaptic organization of afferent inputs are essential factors in determining how synaptic input is integrated by neurons. This information remains elusive for one of the first brainstem neurons involved in processing of the primary auditory signal from the ear, the bushy cells (BCs) of the ventral cochlear nucleus (VCN). Here, we labeled the BC dendritic trees with retrograde tracing techniques to analyze their geometry and synaptic organization after immunofluorescence for excitatory and inhibitory synaptic markers, electron microscopy, morphometry, double tract-tracing methods, and 3D reconstructions. Our study revealed that BC dendrites provide space for a large number of compartmentalized excitatory and inhibitory synaptic interactions. The dendritic inputs on BCs are of cochlear and noncochlear origin, and their proportion and distribution are dependent on the branching pattern and orientation of the dendritic tree in the VCN. Three-dimensional reconstructions showed that BC dendrites branch and cluster with those of other BCs in the core of the VCN. Within the cluster, incoming synaptic inputs establish divergent multiple-contact synapses (dyads and triads) between BCs. Furthermore, neuron-neuron connections including puncta adherentia, sarcoplasmic junctions, and gap junctions are common between BCs, which suggests that these neurons are electrically coupled. Overall, our study demonstrates the existence of a BC network in the rat VCN. This network may establish the neuroanatomical basis for acoustic information processing by individual BCs as well as for enhanced synchronization of the output signal of the VCN.
Figures



References
-
- Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature. 1998;393(6682):268–272. - PubMed
-
- Altschuler RA, Betz H, Parakkal MH, Reeks KA, Wenthold RJ. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986;369(1–2):316–320. - PubMed
-
- Benson TE, Berglund AM, Brown MC. Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol. 1996;365(1):27–41. - PubMed
-
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J Comp Neurol. 1975;160(4):491–506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

