Quantitative analysis of neurons with Kv3 potassium channel subunits, Kv3.1b and Kv3.2, in macaque primary visual cortex
- PMID: 19634181
- PMCID: PMC2925415
- DOI: 10.1002/cne.22111
Quantitative analysis of neurons with Kv3 potassium channel subunits, Kv3.1b and Kv3.2, in macaque primary visual cortex
Abstract
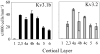
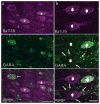
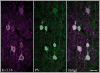
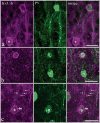
Voltage-gated potassium channels that are composed of Kv3 subunits exhibit distinct electrophysiological properties: activation at more depolarized potentials than other voltage-gated K+ channels and fast kinetics. These channels have been shown to contribute to the high-frequency firing of fast-spiking (FS) GABAergic interneurons in the rat and mouse brain. In the rodent neocortex there are distinct patterns of expression for the Kv3.1b and Kv3.2 channel subunits and of coexpression of these subunits with neurochemical markers, such as the calcium-binding proteins parvalbumin (PV) and calbindin D-28K (CB). The distribution of Kv3 channels and interrelationship with calcium-binding protein expression has not been investigated in primate cortex. We used immunoperoxidase and immunofluorescent labeling and stereological counting techniques to characterize the laminar and cell-type distributions of Kv3-immunoreactive (ir) neurons in macaque V1. We found that across the cortical layers approximately 25% of both Kv3.1b- and Kv3.2-ir neurons are non-GABAergic. In contrast, all Kv3-ir neurons in rodent cortex are GABAergic (Chow et al. [1999] J Neurosci. 19:9332-9345). The putatively excitatory Kv3-ir neurons were mostly located in layers 2, 3, and 4b. Further, the proportion of Kv3-ir neurons that express PV or CB also differs between macaque V1 and rodent cortex. These data indicate that, within the population of cortical neurons, a broader population of neurons, encompassing cells of a wider range of morphological classes may be capable of sustaining high-frequency firing in macaque V1.
Figures









Similar articles
-
Densities and Laminar Distributions of Kv3.1b-, PV-, GABA-, and SMI-32-Immunoreactive Neurons in Macaque Area V1.Cereb Cortex. 2019 May 1;29(5):1921-1937. doi: 10.1093/cercor/bhy072. Cereb Cortex. 2019. PMID: 29668858 Free PMC article.
-
GABAergic and non-GABAergic subpopulations of Kv3.1b-expressing neurons in macaque V2 and MT: laminar distributions and proportion of total neuronal population.Brain Struct Funct. 2020 Apr;225(3):1135-1152. doi: 10.1007/s00429-020-02065-y. Epub 2020 Apr 7. Brain Struct Funct. 2020. PMID: 32266458
-
Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala.Neuroscience. 2006;138(2):537-47. doi: 10.1016/j.neuroscience.2005.11.047. Epub 2006 Jan 18. Neuroscience. 2006. PMID: 16413129
-
Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance.Physiol Rev. 2017 Oct 1;97(4):1431-1468. doi: 10.1152/physrev.00002.2017. Physiol Rev. 2017. PMID: 28904001 Free PMC article. Review.
-
Dysfunctional Parvalbumin Neurons in Schizophrenia and the Pathway to the Clinical Application of Kv3 Channel Modulators.Int J Mol Sci. 2024 Aug 9;25(16):8696. doi: 10.3390/ijms25168696. Int J Mol Sci. 2024. PMID: 39201380 Free PMC article. Review.
Cited by
-
A KCNC1 mutation in epilepsy of infancy with focal migrating seizures produces functional channels that fail to be regulated by PKC phosphorylation.J Neurophysiol. 2021 Aug 1;126(2):532-539. doi: 10.1152/jn.00257.2021. Epub 2021 Jul 7. J Neurophysiol. 2021. PMID: 34232791 Free PMC article.
-
Brain stimulation competes with ongoing oscillations for control of spike timing in the primate brain.PLoS Biol. 2022 May 25;20(5):e3001650. doi: 10.1371/journal.pbio.3001650. eCollection 2022 May. PLoS Biol. 2022. PMID: 35613140 Free PMC article.
-
Local field potentials primarily reflect inhibitory neuron activity in human and monkey cortex.Sci Rep. 2017 Jan 11;7:40211. doi: 10.1038/srep40211. Sci Rep. 2017. PMID: 28074856 Free PMC article.
-
Classification of Cortical Neurons by Spike Shape and the Identification of Pyramidal Neurons.Cereb Cortex. 2021 Oct 1;31(11):5131-5138. doi: 10.1093/cercor/bhab147. Cereb Cortex. 2021. PMID: 34117760 Free PMC article.
-
Neuropixels reveal structure-function relationships in monkey V1 in vivo.bioRxiv [Preprint]. 2025 Jun 25:2025.05.14.653875. doi: 10.1101/2025.05.14.653875. bioRxiv. 2025. PMID: 40463048 Free PMC article. Preprint.
References
-
- Adams I, Brauer K, Arelin C, Hartig W, Fine A, Mader M, Arendt T, Bruckner G. Perineuronal nets in the rhesus monkey and human basal forebrain including basal ganglia. Neurosci. 2001;108(2):285–298. - PubMed
-
- Beaulieu C, Kisvarday Z, Somogyi P, Cynader M, Cowey A. Quantitative distribution of GABA-immunopositive and – immunonegative neurons and synapses in the monkey striate cortex (area 17) Cereb Cortex. 1992;2:295–309. - PubMed
-
- Benson DL, Isackson PJ, Gall CM, Jones EG. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 1992;46(4):825–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY017945/EY/NEI NIH HHS/United States
- R01 EY008300/EY/NEI NIH HHS/United States
- NS045217/NS/NINDS NIH HHS/United States
- T32 HD007430/HD/NICHD NIH HHS/United States
- P30 EY013079/EY/NEI NIH HHS/United States
- R01 NS030989/NS/NINDS NIH HHS/United States
- R03 EY015549/EY/NEI NIH HHS/United States
- EY-08300/EY/NEI NIH HHS/United States
- NS30989/NS/NINDS NIH HHS/United States
- F30 NS071660/NS/NINDS NIH HHS/United States
- R01 NS045217/NS/NINDS NIH HHS/United States
- EY-015549/EY/NEI NIH HHS/United States
- EY-017945/EY/NEI NIH HHS/United States
- EY P031-13079/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

