Radical triplets and suicide inhibition in reactions of 4-thia-D- and 4-thia-L-lysine with lysine 5,6-aminomutase
- PMID: 19634897
- PMCID: PMC2741331
- DOI: 10.1021/bi900828f
Radical triplets and suicide inhibition in reactions of 4-thia-D- and 4-thia-L-lysine with lysine 5,6-aminomutase
Abstract
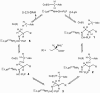
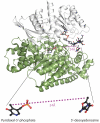
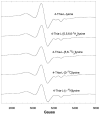
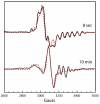
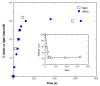
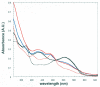
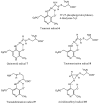
Lysine 5,6-aminomutase (5,6-LAM) catalyzes the interconversions of D- or L-lysine and the corresponding enantiomers of 2,5-diaminohexanoate, as well as the interconversion of L-beta-lysine and l-3,5-diaminohexanoate. The reactions of 5,6-LAM are 5'-deoxyadenosylcobalamin- and pyridoxal-5'-phosphate (PLP)-dependent. Similar to other 5'-deoxyadenosylcobalamin-dependent enzymes, 5,6-LAM is thought to function by a radical mechanism. No free radicals can be detected by electron paramagnetic resonance (EPR) spectroscopy in reactions of 5,6-LAM with D- or L-lysine or with L-beta-lysine. However, the substrate analogues 4-thia-L-lysine and 4-thia-D-lysine undergo early steps in the mechanism to form two radical species that are readily detected by EPR spectroscopy. Cob(II)alamin and 5'-deoxyadenosine derived from 5'-deoxyadenosylcobalamin are also detected. The radicals are proximal to and spin-coupled with low-spin Co(2+) in cob(II)alamin and appear as radical triplets. The radicals are reversibly formed but do not proceed to stable products, so that 4-thia-D- and L-lysine are suicide inhibitors. Inhibition attains equilibrium between the active Michaelis complex and the inhibited radical triplets. The structure of the transient 4-thia-L-lysine radical is analogous to that of the first substrate-related radical in the putative isomerization mechanism. The second, persistent radical is more stable than the transient species and is assigned as a tautomer, in which a C6(H) of the transient radical is transferred to the carboxaldehyde carbon (C4') of PLP. The persistent radical blocks the active site and inhibits the enzyme, but it decomposes very slowly at </=1% of the rate of formation to regenerate the active enzyme. Fundamental differences between reversible suicide inactivation by 4-thia-D- or L-4-lysine and irreversible suicide inactivation by D- or L-lysine are discussed. The observation of the transient radical supports the hypothetical isomerization mechanism.
Figures

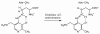


References
-
- Stadtman TC. Lysine metabolism by Clostridia. Adv. Enzymol. 1973;28:413–448.
-
- Tang K-H, Chang CH, Frey PA. Electron transfer in the substrate-dependent suicide inactivation of lysine 5,6-aminomutase. Biochemistry. 2001;40:5190–5199. - PubMed
-
- Chang CH, Frey PA. Cloning, sequencing, heterologous expression, purification, and characterization of adenosylcobalamin-dependent d-lysine 5, 6-aminomutase from Clostridium sticklandii. J. Biol. Chem. 2000;275:106–114. - PubMed
-
- Tang K-H, Harms A, Frey PA. Identification of a novel pyridoxal 5′-phosphate binding site in adenosylcobalamin-dependent lysine 5,6-aminomutase from Porphyromonas gingivalis. Biochemistry. 2002;41:8767–8776. - PubMed
-
- Baker JJ, van der Drift C, Stadtman TC. Purification and properties of -lysine mutase, a pyridoxal phosphate and B 12 coenzyme dependent enzyme. Biochemistry. 1973;12:1054–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

