Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits
- PMID: 19634929
- PMCID: PMC2791672
- DOI: 10.1037/a0016503
Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits
Abstract
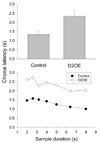
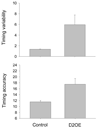
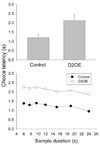
Increased striatal dopamine D2 receptor activity is thought to contribute to the pathophysiology of schizophrenia. To model this condition in mice, Kellendonk et al. (2006) generated transgenic mice that selectively overexpress the D2 receptor in striatum (D2OE). Drew et al. (2007) reported that D2OE mice display deficits in interval timing and motivation. The present study further explored the impaired timing in D2OE mice. Experiment 1 assessed the role of motivation in producing timing deficits in the peak procedure and found that performance in D2OE mice was improved by increasing motivation. In addition, performance was impaired in control mice when motivation was decreased. In Experiment 2, we found that D2OE mice have no timing impairment when tested using the bisection task, a procedure in which the measure of timing performance is less influenced by motivation to respond. In Experiment 3, we also used the bisection task and found selective impairment in timing of long durations in D2OE mice. These results suggest that striatal D2 overexpression impairs timing by decreasing motivation and through its impact on working memory and/or sustained attention.
2009 APA, all rights reserved
Figures





Similar articles
-
The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia.Behav Neurosci. 2015 Jun;129(3):292-9. doi: 10.1037/bne0000051. Epub 2015 Apr 27. Behav Neurosci. 2015. PMID: 25914923 Free PMC article.
-
Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing.J Neurosci. 2007 Jul 18;27(29):7731-9. doi: 10.1523/JNEUROSCI.1736-07.2007. J Neurosci. 2007. PMID: 17634367 Free PMC article.
-
Selective overexpression of dopamine D3 receptors in the striatum disrupts motivation but not cognition.Biol Psychiatry. 2014 Nov 15;76(10):823-31. doi: 10.1016/j.biopsych.2013.11.023. Epub 2013 Dec 5. Biol Psychiatry. 2014. PMID: 24387821 Free PMC article.
-
Timing as a window on cognition in schizophrenia.Neuropharmacology. 2012 Mar;62(3):1175-81. doi: 10.1016/j.neuropharm.2011.04.014. Epub 2011 Apr 21. Neuropharmacology. 2012. PMID: 21530549 Free PMC article. Review.
-
[Cognition, schizophrenia and the effect of antipsychotics].Encephale. 2006 May-Jun;32(3 Pt 1):341-50. doi: 10.1016/s0013-7006(06)76162-0. Encephale. 2006. PMID: 16840928 Review. French.
Cited by
-
The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia.Behav Neurosci. 2015 Jun;129(3):292-9. doi: 10.1037/bne0000051. Epub 2015 Apr 27. Behav Neurosci. 2015. PMID: 25914923 Free PMC article.
-
Dopamine D2 receptors in nucleus accumbens cholinergic interneurons increase impulsive choice.bioRxiv [Preprint]. 2023 Jan 20:2023.01.20.524596. doi: 10.1101/2023.01.20.524596. bioRxiv. 2023. Update in: Neuropsychopharmacology. 2023 Aug;48(9):1309-1317. doi: 10.1038/s41386-023-01608-1. PMID: 36711450 Free PMC article. Updated. Preprint.
-
The instrumental role of operant paradigms in translational psychiatric research: Insights from a maternal immune activation model of schizophrenia risk.J Exp Anal Behav. 2022 May;117(3):560-575. doi: 10.1002/jeab.753. Epub 2022 Mar 23. J Exp Anal Behav. 2022. PMID: 35319781 Free PMC article. Review.
-
Altered fronto-striatal functions in the Gdi1-null mouse model of X-linked Intellectual Disability.Neuroscience. 2017 Mar 6;344:346-359. doi: 10.1016/j.neuroscience.2016.12.043. Epub 2017 Jan 3. Neuroscience. 2017. PMID: 28057534 Free PMC article.
-
Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels.J Neurosci. 2012 Feb 15;32(7):2398-409. doi: 10.1523/JNEUROSCI.6056-11.2012. J Neurosci. 2012. PMID: 22396414 Free PMC article.
References
-
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. American Journal of Psychiatry. 1998;155:761–767. - PubMed
-
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. - PubMed
-
- Buhusi CV, Meck WH. Timing for the absence of a stimulus: The gap paradigm reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:305–322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

