Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders
- PMID: 19634955
- PMCID: PMC2795638
- DOI: 10.1037/a0016227
Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders
Abstract
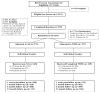
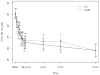
The authors compared the effectiveness of the Seeking Safety group, cognitive-behavioral treatment for substance use disorder and posttraumatic stress disorder (PTSD), to an active comparison health education group (Women's Health Education [WHE]) within the National Institute on Drug Abuse's Clinical Trials Network. The authors randomized 353 women to receive 12 sessions of Seeking Safety (M = 6.2 sessions) or WHE (M = 6.0 sessions) with follow-up assessment 1 week and 3, 6, and 12 months posttreatment. Primary outcomes were the Clinician Administered PTSD Scale (CAPS), the PTSD Symptom Scale-Self Report (PSS-SR), and a substance use inventory (self-reported abstinence and percentage of days of use over 7 days). Intention-to-treat analysis showed large, clinically significant reductions in CAPS and PSS-SR symptoms (d = 1.94 and 1.12, respectively) but no reliable difference between conditions. Substance use outcomes were not significantly different over time between the two treatments and at follow-up showed no significant change from baseline. Study results do not favor Seeking Safety over WHE as an adjunct to substance use disorder treatment for women with PTSD and reflect considerable opportunity to improve clinical outcomes in community-based treatments for these co-occurring conditions.
Trial registration: ClinicalTrials.gov NCT00078156.
Figures
References
-
- Amaro H, Dai J, Arevalo S, Acevedo A, Matsumoto A, Nieves R, et al. Effects of integrated trauma treatment on outcomes in a racially/ethnically diverse sample of women in urban community-based substance abuse treatment. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2007;84(4):508–522. - PMC - PubMed
-
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text revision.
-
- Blake DB, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. - PubMed
-
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ. Psychological morbidity associated with motor vehicle accidents. Behavior Research and Therapy. 1994;32:283–290. - PubMed
-
- Brady KT, Buonopane A, Draper JC, Kalbag AS, Levin FR, McCance-Katz EF, et al. The most critical contemporary unresolved issues associated with the pharmacotherapy treatment of substance users. Substance Use & Misuse. 2005;40(13):2043–2048. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U10 DA013046/DA/NIDA NIH HHS/United States
- U10 DA13038/DA/NIDA NIH HHS/United States
- U10 DA013714/DA/NIDA NIH HHS/United States
- U10 DA13714/DA/NIDA NIH HHS/United States
- U10 DA013732/DA/NIDA NIH HHS/United States
- U10 DA13732/DA/NIDA NIH HHS/United States
- U10 DA013727/DA/NIDA NIH HHS/United States
- U10 DA013720/DA/NIDA NIH HHS/United States
- U10 DA013038/DA/NIDA NIH HHS/United States
- U10 DA013035/DA/NIDA NIH HHS/United States
- U10 DA13727/DA/NIDA NIH HHS/United States
- K24 DA022412/DA/NIDA NIH HHS/United States
- U10 DA13035/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

