A Dictyostelium chalone uses G proteins to regulate proliferation
- PMID: 19635129
- PMCID: PMC2726123
- DOI: 10.1186/1741-7007-7-44
A Dictyostelium chalone uses G proteins to regulate proliferation
Abstract
Background: Several studies have shown that organ size, and the proliferation of tumor metastases, may be regulated by negative feedback loops in which autocrine secreted factors called chalones inhibit proliferation. However, very little is known about chalones, and how cells sense them. We previously identified two secreted proteins, AprA and CfaD, which act as chalones in Dictyostelium. Cells lacking AprA or CfaD proliferate faster than wild-type cells, and adding recombinant AprA or CfaD to cells slows their proliferation.
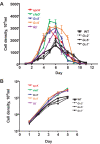
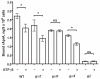
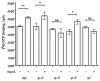
Results: We show here that cells lacking the G protein components Galpha8, Galpha9, and Gbeta proliferate faster than wild-type cells despite secreting normal or high levels of AprA and CfaD. Compared with wild-type cells, the proliferation of galpha8-, galpha9- and gbeta- cells are only weakly inhibited by recombinant AprA (rAprA). Like AprA and CfaD, Galpha8 and Gbeta inhibit cell proliferation but not cell growth (the rate of increase in mass and protein per nucleus), whereas Galpha9 inhibits both proliferation and growth. galpha8- cells show normal cell-surface binding of rAprA, whereas galpha9- and gbeta- cells have fewer cell-surface rAprA binding sites, suggesting that Galpha9 and Gbeta regulate the synthesis or processing of the AprA receptor. Like other ligands that activate G proteins, rAprA induces the binding of [3H]GTP to membranes, and GTPgammaS inhibits the binding of rAprA to membranes. Both AprA-induced [3H]GTP binding and the GTPgammaS inhibition of rAprA binding require Galpha8 and Gbeta but not Galpha9. Like aprA- cells, galpha8- cells have reduced spore viability.
Conclusion: This study shows that Galpha8 and Gbeta are part of the signal transduction pathway used by AprA to inhibit proliferation but not growth in Dictyostelium, whereas Galpha9 is part of a differealnt pathway that regulates both proliferation and growth, and that a chalone signal transduction pathway uses G proteins.
Figures
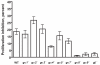


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

