Immunohistochemical and biochemical characteristics of BSE and CWD in experimentally infected European red deer (Cervus elaphus elaphus)
- PMID: 19635142
- PMCID: PMC2724422
- DOI: 10.1186/1746-6148-5-26
Immunohistochemical and biochemical characteristics of BSE and CWD in experimentally infected European red deer (Cervus elaphus elaphus)
Abstract
Background: The cause of the bovine spongiform encephalopathy (BSE) epidemic in the United Kingdom (UK) was the inclusion of contaminated meat and bone meal in the protein rations fed to cattle. Those rations were not restricted to cattle but were also fed to other livestock including farmed and free living deer. Although there are no reported cases to date of natural BSE in European deer, BSE has been shown to be naturally or experimentally transmissible to a wide range of different ungulate species. Moreover, several species of North America's cervids are highly susceptible to chronic wasting disease (CWD), a transmissible spongiform encephalopathy (TSE) that has become endemic. Should BSE infection have been introduced into the UK deer population, the CWD precedent could suggest that there is a danger for spread and maintenance of the disease in both free living and captive UK deer populations. This study compares the immunohistochemical and biochemical characteristics of BSE and CWD in experimentally-infected European red deer (Cervus elpahus elaphus).
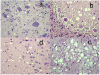
Results: After intracerebral or alimentary challenge, BSE in red deer more closely resembled natural infection in cattle rather than experimental BSE in small ruminants, due to the lack of accumulation of abnormal PrP in lymphoid tissues. In this respect it was different from CWD, and although the neuropathological features of both diseases were similar, BSE could be clearly differentiated from CWD by immunohistochemical and Western blotting methods currently in routine use.
Conclusion: Red deer are susceptible to both BSE and CWD infection, but the resulting disease phenotypes are distinct and clearly distinguishable.
Figures









References
-
- Wilesmith JW, Wells GAH, Cranwell MP, Ryan JBN. BSE: Epidemiological Studies. Vet Record. 1988;123:638–644. - PubMed
-
- Jeffrey M, Wells GA. Spongiform encephalopathy in a nyala (Tragelaphus angasi) Vet Pathol. 1988;25:398–399. - PubMed
-
- Kirkwood JK, Cunningham AA. Epidemiologic Observations on Spongiform Encephalopathies in Captive Wild Animals in the British-Isles. Veterinary Record. 1994;135:296–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

