Relative bioavailability of iron and folic acid from a new powdered supplement compared to a traditional tablet in pregnant women
- PMID: 19635145
- PMCID: PMC2724426
- DOI: 10.1186/1471-2393-9-33
Relative bioavailability of iron and folic acid from a new powdered supplement compared to a traditional tablet in pregnant women
Abstract
Background: Deficiencies of iron and folic acid during pregnancy can lead to adverse outcomes for the fetus, thus supplements are recommended. Adherence to current tablet-based supplements is documented to be poor. Recently a powdered form of micronutrients has been developed which may decrease side-effects and thus improve adherence. However, before testing the efficacy of the supplement as an alternate choice for supplementation during pregnancy, the bioavailability of the iron needs to be determined. Our objective was to measure the relative bioavailability of iron and folic acid from a powdered supplement that can be sprinkled on semi-solid foods or beverages versus a traditional tablet supplement in pregnant women.
Methods: Eighteen healthy pregnant women (24 - 32 weeks gestation) were randomized to receive the supplements in a crossover design. Following ingestion of each supplement, the changes (over baseline) in serum iron and folate over 8 hours were determined. The powdered supplement contained 30 mg of iron as micronized dispersible ferric pyrophosphate with an emulsifier coating and 600 mug folic acid; the tablet contained 27 mg iron from ferrous fumarate and 1000 mug folic acid.
Results: Overall absorption of iron from the powdered supplement was significantly lower than the tablet (p = 0.003). There was no difference in the overall absorption of folic acid between supplements. Based on the differences in the area under the curve and doses, the relative bioavailability of iron from powdered supplement was lower than from the tablet (0.22).
Conclusion: The unexpected lower bioavailability of iron from the powdered supplement is contrary to previously published reports. However, since pills and capsules are known to be poorly accepted by some women during pregnancy, it is reasonable to continue to explore alternative micronutrient delivery systems and forms of iron for this purpose.
Trial registration: ClinicalTrials.gov NCT00789490.
Figures
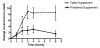

References
-
- Viteri FE, Berger J. Importance of pre-pregnancy and pregnancy iron status: can long-term weekly preventive iron and folic acid supplementation achieve desirable and safe status? Nutr Rev. 2005;63:S65–76. - PubMed
-
- Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016. - PubMed
-
- Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295S–1303S. - PubMed
-
- Chalmers B, Dzakpasu S, Heaman M, Kaczorowski J. The Canadian maternity experiences survey: an overview of findings. J Obstet Gynaecol Can. 2008;30:217–228. - PubMed
-
- Melamed N, Ben-Haroush A, Kaplan B, Yogev Y. Iron supplementation in pregnancy – does the preparation matter? Arch Gynecol Obstet. 2007;276:601–604. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

