Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10
- PMID: 19635168
- PMCID: PMC2722575
- DOI: 10.1186/1471-2121-10-56
Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10
Abstract
Background: The Dbl family guanine nucleotide exchange factor ARHGEF10 was originally identified as the product of the gene associated with slowed nerve-conduction velocities of peripheral nerves. However, the function of ARHGEF10 in mammalian cells is totally unknown at a molecular level. ARHGEF10 contains no distinctive functional domains except for tandem Dbl homology-pleckstrin homology and putative transmembrane domains.
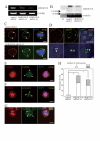
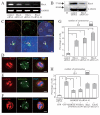
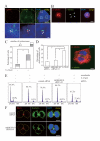
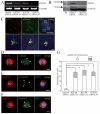
Results: Here we show that RhoA is a substrate for ARHGEF10. In both G1/S and M phases, ARHGEF10 was localized in the centrosome in adenocarcinoma HeLa cells. Furthermore, RNA interference-based knockdown of ARHGEF10 resulted in multipolar spindle formation in M phase. Each spindle pole seems to contain a centrosome consisting of two centrioles and the pericentriolar material. Downregulation of RhoA elicited similar phenotypes, and aberrant mitotic spindle formation following ARHGEF10 knockdown was rescued by ectopic expression of constitutively activated RhoA. Multinucleated cells were not increased upon ARHGEF10 knockdown in contrast to treatment with Y-27632, a specific pharmacological inhibitor for the RhoA effector kinase ROCK, which induced not only multipolar spindle formation, but also multinucleation. Therefore, unregulated centrosome duplication rather than aberration in cytokinesis may be responsible for ARHGEF10 knockdown-dependent multipolar spindle formation. We further isolated the kinesin-like motor protein KIF3B as a binding partner of ARHGEF10. Knockdown of KIF3B again caused multipolar spindle phenotypes. The supernumerary centrosome phenotype was also observed in S phase-arrested osteosarcoma U2OS cells when the expression of ARHGEF10, RhoA or KIF3B was abrogated by RNA interference.
Conclusion: Collectively, our results suggest that a novel RhoA-dependent signaling pathway under the control of ARHGEF10 has a pivotal role in the regulation of the cell division cycle. This pathway is not involved in the regulation of cytokinesis, but instead may regulate centrosome duplication. The kinesin-like motor protein KIF3B may modulate the ARHGEF10-RhoA pathway through the binding to ARHGEF10.
Figures



Similar articles
-
Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10).J Biol Chem. 2011 Aug 26;286(34):29511-20. doi: 10.1074/jbc.M111.236810. Epub 2011 Jun 30. J Biol Chem. 2011. PMID: 21719701 Free PMC article.
-
P190RhoGAP prevents mitotic spindle fragmentation and is required to activate Aurora A kinase at acentriolar poles.Chromosoma. 2018 Sep;127(3):375-386. doi: 10.1007/s00412-018-0670-0. Epub 2018 Apr 14. Chromosoma. 2018. PMID: 29656322 Free PMC article.
-
Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle.Mol Biol Cell. 2009 Mar;20(5):1428-40. doi: 10.1091/mbc.e08-01-0001. Epub 2009 Jan 7. Mol Biol Cell. 2009. PMID: 19129481 Free PMC article.
-
Spatiotemporal Regulation of RhoA during Cytokinesis.Curr Biol. 2018 May 7;28(9):R570-R580. doi: 10.1016/j.cub.2018.03.045. Curr Biol. 2018. PMID: 29738735 Free PMC article. Review.
-
Involvement of ARHGEF10, GEF for RhoA, in Rab6/Rab8-mediating membrane traffic.Small GTPases. 2019 May;10(3):169-177. doi: 10.1080/21541248.2017.1302550. Epub 2017 Apr 27. Small GTPases. 2019. PMID: 28448737 Free PMC article. Review.
Cited by
-
Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors.Nat Commun. 2017 Oct 31;8(1):1221. doi: 10.1038/s41467-017-01355-0. Nat Commun. 2017. PMID: 29089486 Free PMC article.
-
Tumor necrosis factor-induced ArhGEF10 selectively activates RhoB contributing to human microvascular endothelial cell tight junction disruption.FASEB J. 2021 Jun;35(6):e21627. doi: 10.1096/fj.202002783RR. FASEB J. 2021. PMID: 33948992 Free PMC article.
-
Rho GTPase-independent regulation of mitotic progression by the RhoGEF Net1.Mol Biol Cell. 2013 Sep;24(17):2655-67. doi: 10.1091/mbc.E13-01-0061. Epub 2013 Jul 17. Mol Biol Cell. 2013. PMID: 23864709 Free PMC article.
-
Genomic convergence of locus-based GWAS meta-analysis identifies AXIN1 as a novel Parkinson's gene.Immunogenetics. 2018 Sep;70(9):563-570. doi: 10.1007/s00251-018-1068-0. Epub 2018 Jun 19. Immunogenetics. 2018. PMID: 29923028
-
Dock8 interacts with Nck1 in mediating Schwann cell precursor migration.Biochem Biophys Rep. 2016 Mar 24;6:113-123. doi: 10.1016/j.bbrep.2016.03.013. eCollection 2016 Jul. Biochem Biophys Rep. 2016. PMID: 28955869 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

