Rationale and design of the oral HEMe iron polypeptide Against Treatment with Oral Controlled Release Iron Tablets trial for the correction of anaemia in peritoneal dialysis patients (HEMATOCRIT trial)
- PMID: 19635169
- PMCID: PMC2723098
- DOI: 10.1186/1471-2369-10-20
Rationale and design of the oral HEMe iron polypeptide Against Treatment with Oral Controlled Release Iron Tablets trial for the correction of anaemia in peritoneal dialysis patients (HEMATOCRIT trial)
Abstract
Background: The main hypothesis of this study is that oral heme iron polypeptide (HIP; Proferrin ES) administration will more effectively augment iron stores in erythropoietic stimulatory agent (ESA)-treated peritoneal dialysis (PD) patients than conventional oral iron supplementation (Ferrogradumet).
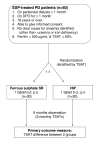
Methods: Inclusion criteria are peritoneal dialysis patients treated with darbepoietin alpha (DPO; Aranesp(R), Amgen) for >or= 1 month. Patients will be randomized 1:1 to receive either slow-release ferrous sulphate (1 tablet twice daily; control) or HIP (1 tablet twice daily) for a period of 6 months. The study will follow an open-label design but outcome assessors will be blinded to study treatment. During the 6-month study period, haemoglobin levels will be measured monthly and iron studies (including transferring saturation [TSAT] measurements) will be performed bi-monthly. The primary outcome measure will be the difference in TSAT levels between the 2 groups at the end of the 6 month study period, adjusted for baseline values using analysis of covariance (ANCOVA). Secondary outcome measures will include serum ferritin concentration, haemoglobin level, DPO dosage, Key's index (DPO dosage divided by haemoglobin concentration), and occurrence of adverse events (especially gastrointestinal adverse events).
Discussion: This investigator-initiated multicentre study has been designed to provide evidence to help nephrologists and their peritoneal dialysis patients determine whether HIP administration more effectively augments iron stores in ESP-treated PD patients than conventional oral iron supplementation.
Trial registration: Australia New Zealand Clinical Trials Registry number ACTRN12609000432213.
Figures
References
-
- Eschbach JW Jr, Funk D, Adamson J, Kuhn I, Scribner BH, Finch CA. Erythropoiesis in patients with renal failure undergoing chronic dialysis. N Engl J Med. 1967;276:653–658. - PubMed
-
- Benz RL, Pressman MR, Hovick ET, Peterson DD. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study) Am J Kidney Dis. 1999;34:1089–1095. doi: 10.1016/S0272-6386(99)70015-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical