Development of bioactive peptide amphiphiles for therapeutic cell delivery
- PMID: 19635599
- PMCID: PMC2787676
- DOI: 10.1016/j.actbio.2009.07.031
Development of bioactive peptide amphiphiles for therapeutic cell delivery
Abstract
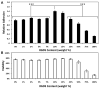
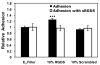
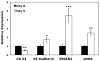
There is great clinical interest in cell-based therapies for ischemic tissue repair in cardiovascular disease. However, the regenerative potential of these therapies is limited due to poor cell viability and minimal retention following application. We report here the development of bioactive peptide amphiphile nanofibers displaying the fibronectin-derived RGDS cell adhesion epitope as a scaffold for therapeutic delivery of bone marrow derived stem and progenitor cells. When grown on flat substrates, a binary peptide amphiphile system consisting of 10 wt.% RGDS-containing molecules and 90wt.% negatively charged diluent molecules was found to promote optimal cell adhesion. This binary system enhanced adhesion 1.4-fold relative to substrates composed of only the non-bioactive diluent. Additionally, no enhancement was found upon scrambling the epitope and adhesion was no longer enhanced upon adding soluble RGDS to the cell media, indicating RGDS-specific adhesion. When encapsulated within self-assembled scaffolds of the binary RGDS nanofibers in vitro, cells were found to be viable and proliferative, increasing in number by 5.5 times after only 5 days, an effect again lost upon adding soluble RGDS. Cells encapsulated within a non-bioactive scaffold and those within a binary scaffold with scrambled epitope showed minimal viability and no proliferation. Cells encapsulated within this RGDS nanofiber gel also increase in endothelial character, evident by a decrease in the expression of CD34 paired with an increase in the expression of endothelial-specific markers VE-Cadherin, VEGFR2 and eNOS after 5 days. In an in vivo study, nanofibers and luciferase-expressing cells were co-injected subcutaneously in a mouse model. The binary RGDS material supported these cells in vivo, evident by a 3.2-fold increase in bioluminescent signal attributable to viable cells; this suggests the material has an anti-apoptotic and/or proliferative effect on the transplanted bone marrow cells. We conclude that the binary RGDS-presenting nanofibers developed here demonstrate enhanced viability, proliferation and adhesion of associated bone marrow derived stem and progenitor cells. This study suggests potential for this material as a scaffold to overcome current limitations of stem cell therapies for ischemic diseases.
Figures

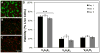


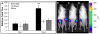
Republished in
-
Reprint of: Development of bioactive peptide amphiphiles for therapeutic cell delivery.Acta Biomater. 2015 Sep;23 Suppl:S42-51. doi: 10.1016/j.actbio.2015.07.018. Acta Biomater. 2015. PMID: 26235345
References
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–146. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb 14;275(5302):964–967. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999 Aug 6;85(3):221–228. - PubMed
-
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1210–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL077428/HL/NHLBI NIH HHS/United States
- 1R01-EB003806-04/EB/NIBIB NIH HHS/United States
- R01 HL080137/HL/NHLBI NIH HHS/United States
- R01 HL057516/HL/NHLBI NIH HHS/United States
- HL-57516/HL/NHLBI NIH HHS/United States
- HL-53354/HL/NHLBI NIH HHS/United States
- P01 HL066957/HL/NHLBI NIH HHS/United States
- 5T90-DA022881/DA/NIDA NIH HHS/United States
- R37 HL053354/HL/NHLBI NIH HHS/United States
- R01 EB003806/EB/NIBIB NIH HHS/United States
- HL-63414/HL/NHLBI NIH HHS/United States
- R01 HL053354/HL/NHLBI NIH HHS/United States
- R01 HL063414/HL/NHLBI NIH HHS/United States
- HL-80137/HL/NHLBI NIH HHS/United States
- T90 DA022881/DA/NIDA NIH HHS/United States
- HL-77428/HL/NHLBI NIH HHS/United States
- P01HL-66957/HL/NHLBI NIH HHS/United States
- R01 HL095874/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

