The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study
- PMID: 19635719
- PMCID: PMC2732898
- DOI: 10.1136/ard.2009.108936
The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study
Abstract
Background: Recent studies suggest that blockade of the NLRP3 (cryopyrin) inflammasome interleukin 1beta (IL1beta) pathway may offer a new treatment strategy for gout.
Objective: To explore the potential utility of rilonacept (IL1 Trap) in patients with chronic active gouty arthritis in a proof-of-concept study.
Methods: This 14-week, multicentre, non-randomised, single-blind, monosequence crossover study of 10 patients with chronic active gouty arthritis included a placebo run-in (2 weeks), active rilonacept treatment (6 weeks) and a 6-week post-treatment follow-up.
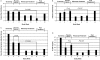
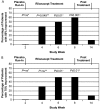
Results: Rilonacept was generally well tolerated. No deaths and no serious adverse events occurred during the study. One patient withdrew owing to an injection-site reaction. Patients' self-reported median pain visual analogue scale scores significantly decreased from week 2 (after the placebo run-in) to week 4 (2 weeks of rilonacept) (5.0 to 2.8; p<0.049), with sustained improvement at week 8 (1.3; p<0.049); 5 of 10 patients reported at least a 75% improvement. Median symptom-adjusted and severity-adjusted joint scores were significantly decreased. High-sensitivity C-reactive protein levels fell significantly.
Conclusions: This proof-of-concept study demonstrated that rilonacept is generally well tolerated and may offer therapeutic benefit in reducing pain in patients with chronic refractory gouty arthritis, supporting the need for larger, randomised, controlled studies of IL1 antagonism such as with rilonacept for this clinical indication.
Conflict of interest statement
Figures
Comment in
-
A magic bullet for gout?Ann Rheum Dis. 2009 Oct;68(10):1517-9. doi: 10.1136/ard.2009.112508. Ann Rheum Dis. 2009. PMID: 19748918 No abstract available.
References
-
- Edwards NL. Treatment-failure gout: A moving target. Arthritis Rheum 2008;58:2587–90 - PubMed
-
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med 2003;349:1647–55 - PubMed
-
- Sundy JS, Becker MA, Baraf HS, et al. Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum 2008;58:2882–91 - PubMed
-
- Becker MA, Schumacher HR, Benjamin KL, et al. Quality of life and disability in patients with treatment-failure gout. J Rheumatol 2009;36:1041–8 - PubMed
-
- Taylor WJ, Schumacher HR, Jr, Singh JA, et al. Assesment of outcome in clinical trials of gout – a review of current measures. Rheumatology (Oxford) 2007;46:1751–76 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

