Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupinus albus L., and Lupinus mutabilis Sweet
- PMID: 19635747
- PMCID: PMC2724698
- DOI: 10.1093/jxb/erp186
Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupinus albus L., and Lupinus mutabilis Sweet
Abstract
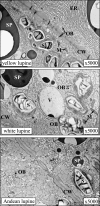
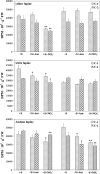
A comparative study was carried out on the dynamics of lipid accumulation in developing seeds of three lupine species. Lupine seeds differ in lipid content; yellow lupine (Lupinus luteus L.) seeds contain about 6%, white lupine (Lupinus albus L.) 7-14%, and Andean lupine (Lupinus mutabilis Sweet) about 20% of lipids by dry mass. Cotyledons from developing seeds were isolated and cultured in vitro for 96 h on Heller medium with 60 mM sucrose (+S) or without sucrose (-S). Each medium was additionally enriched with 35 mM asparagine or 35 mM NaNO3. Asparagine caused an increase in protein accumulation and simultaneously decreased the lipid content, but nitrate increased accumulation of both protein and lipid. Experiments with [1-14C]acetate and [2-14C]acetate showed that the decrease in lipid accumulation in developing lupine seeds resulted from exhaustion of lipid precursors rather than from degradation or modification of the enzymatic apparatus. The carbon atom from the C-1 position of acetate was liberated mainly as CO2, whereas the carbon atom from the C-2 position was preferentially used in anabolic pathways. The dominant phospholipid in the investigated lupine seed storage organs was phosphatidylcholine. The main fatty acid in yellow lupine cotyledons was linoleic acid, in white lupine it was oleic acid, and in Andean lupine it was both linoleic and oleic acids. The relationship between stimulation of lipid and protein accumulation by nitrate in developing lupine cotyledons and enhanced carbon flux through glycolysis caused by the inorganic nitrogen form is discussed.
Figures










Similar articles
-
Storage lipids as a source of carbon skeletons for asparagine synthesis in germinating seeds of yellow lupine (Lupinus luteus L.).J Plant Physiol. 2010 Jun 15;167(9):717-24. doi: 10.1016/j.jplph.2009.12.010. Epub 2010 Feb 18. J Plant Physiol. 2010. PMID: 20170979
-
Nitrate simultaneously enhances lipid and protein accumulation in developing yellow lupin cotyledons cultured in vitro, but not under field conditions.J Plant Physiol. 2017 Sep;216:26-34. doi: 10.1016/j.jplph.2017.03.021. Epub 2017 May 21. J Plant Physiol. 2017. PMID: 28558332
-
A transfer of carbon atoms from fatty acids to sugars and amino acids in yellow lupine (Lupinus luteus L.) seedlings.J Plant Physiol. 2003 May;160(5):539-45. doi: 10.1078/0176-1617-00763. J Plant Physiol. 2003. PMID: 12806783
-
Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges.Molecules. 2022 Dec 5;27(23):8557. doi: 10.3390/molecules27238557. Molecules. 2022. PMID: 36500649 Free PMC article. Review.
-
The pivotal role of glutamate dehydrogenase (GDH) in the mobilization of N and C from storage material to asparagine in germinating seeds of yellow lupine.J Plant Physiol. 2008 Feb;165(2):149-58. doi: 10.1016/j.jplph.2006.12.010. Epub 2007 Jun 12. J Plant Physiol. 2008. PMID: 17566603 Review.
Cited by
-
Transcriptome profiling identifies ABA mediated regulatory changes towards storage filling in developing seeds of castor bean (Ricinus communis L.).Cell Biosci. 2014 Jun 30;4:33. doi: 10.1186/2045-3701-4-33. eCollection 2014. Cell Biosci. 2014. PMID: 25061509 Free PMC article.
-
Regulatory mechanism of carbohydrate metabolism pathways on oil biosynthesis of oil plant Symplocos paniculata.Front Plant Sci. 2025 Feb 6;16:1452533. doi: 10.3389/fpls.2025.1452533. eCollection 2025. Front Plant Sci. 2025. PMID: 39980488 Free PMC article.
-
Regulatory Effects of ABA and GA on the Expression of Conglutin Genes and LAFL Network Genes in Yellow Lupine (Lupinus luteus L.) Seeds.Int J Mol Sci. 2023 Aug 3;24(15):12380. doi: 10.3390/ijms241512380. Int J Mol Sci. 2023. PMID: 37569754 Free PMC article.
-
Genetic mapping and functional genomics of soybean seed protein.Mol Breed. 2023 Apr 12;43(4):29. doi: 10.1007/s11032-023-01373-5. eCollection 2023 Apr. Mol Breed. 2023. PMID: 37313523 Free PMC article.
-
Experimental Evidence for Seed Metabolic Allometry in Barrel Medic (Medicago truncatula Gaertn.).Int J Mol Sci. 2022 Jul 30;23(15):8484. doi: 10.3390/ijms23158484. Int J Mol Sci. 2022. PMID: 35955618 Free PMC article.
References
-
- Allen CF, Good P, Davis HF, Chisum P, Fowler SD. Methodology for the separation of plant lipids and application to spinach leaf and chloroplast lamellae. Journal of the American Oil Chemists Society. 1966;43:223–230.
-
- Allen DK, Ohlrogge JB, Shachar-Hill Y. The role of light in soybean seed filling metabolism. The Plant Journal. 2009;58:220–234. - PubMed
-
- Alonso AP, Goffman FD, Ohlrogge JB, Shachar-Hill Y. Carbon conversion efficiency and central metabolic fluxes in developing sunflower (Helianthus annuus L.) embryos. The Plant Journal. 2007;52:296–308. - PubMed
-
- Ames DN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology. 1966;8:115–118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

